Challenges in Initiating a Cerebral Aneurysm Coiling Programme in a Small Centre: Our Experience after the First 100 Cases
PERSPECTIVE
Challenges in Initiating a Cerebral Aneurysm Coiling Programme in a Small Centre: Our Experience after the First 100 Cases
C Woodworth1, V Linehan2, N Hache1, R Bhatia1, P Bartlett1
1 Discipline of Radiology, Faculty of Medicine, Memorial University of Newfoundland, Canada
2 Department of Diagnostic Radiology, Faculty of Medicine, Dalhousie University, Canada
Correspondence: Dr V Linehan, Department of Diagnostic Radiology, Faculty of Medicine, Dalhousie University, Canada. Email: victoria.linehan@dal.ca
Submitted: 27 Mar 2021; Accepted: 17 Jun 2021.
Contributors: CW, NH, RB and PB designed the study. CW and VL acquired and analysed the data. All authors drafted the manuscript and critically revised the manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: To verify the quality of our newly implemented neurointerventional programme, we obtained local ethics approval to initiate a retrospective review of all patients who underwent cerebral coiling from March 2013 until December 2017.
Abstract
After completing the 100th intracranial aneurysm coiling at our site in 2017, we reflect on the challenges of
implementing a new neurointerventional radiology programme in a small tertiary care centre. Our radiology group
is the sole provider of cerebral coiling for a population of approximately 500,000, first offering this procedure in
March 2013. Given the challenges that we encountered while establishing this programme, we wish to share lessons
learned about resource advocacy, early involvement of key stakeholders, and timely programme introduction to help
others facing similar needs.
Key Words: Aneurysm, ruptured; Embolization, therapeutic; Intracranial aneurysm; Radiology
中文摘要
在小型醫療中心開始腦動脈彈簧圈栓塞術面臨的挑戰:最初100例後我們的經驗
C Woodworth、V Linehan、N Hache、R Bhatia、P Bartlett
2017年在我們的醫院完成第100例顱內動脈瘤彈簧圈栓塞手術後,我們回顧在小型三級醫療中心實施新的神經介入放射學計劃所面臨的挑戰。於2013年3月首次開始,我們的放射科是為大約500,000人口提供顱內動脈彈簧圈栓塞手術的唯一醫療機構。鑑於我們在建立該計劃時遇到的挑戰,我們希望分享有關爭取資源、早期溝通相關人士、並及時把我們的計劃介紹給面臨類似需求機構的經驗。
BACKGROUND
Most commonly found at arterial bifurcations in the circle of Willis, intracranial aneurysms have a prevalence of
2% to 3%.[1] Rupture causes subarachnoid haemorrhage,
which is associated with severe neurological impairment
and a 30% to 40% fatality rate.[2] Prompt treatment of
ruptured intracranial aneurysms by surgical clipping or
endovascular coiling reduces morbidity and mortality by
decreasing rebleeding and vasospasm.[3] [4]
The first patient was treated with endovascular therapy in 1990,[5] with the United States Food and Drug
Administration approval of the Guglielmi detachable
coil in 1995. Endovascular coiling became the preferred
treatment in 2002 when the International Subarachnoid
Aneurysm Trial showed a significant benefit of
endovascular treatment, with a 23.9% reduction in the
relative risk of death or disability at 1 year compared to
surgical clipping.[6]
Despite becoming one of the standard treatments for
ruptured cerebral aneurysms, this interventional service
is not uniformly available. Before a cerebral coiling
service was established at the Health Sciences Centre,
a small tertiary centre in the Canadian province of
Newfoundland, all ruptured aneurysms from within
the local population of 500,000 had to be transferred
to a larger centre in the neighbouring province of Nova
Scotia (1.5 hours by air) for endovascular treatment. In
2003, our Chief of Neurosurgery wrote a formal letter to
our radiology department stating that it was ‘imperative’
that endovascular coiling of intracranial aneurysms ‘be
started as soon as possible at our institution’ to reduce
unnecessary treatment delays. Therefore, we began the
daunting task of implementing a new neurointerventional
service at our centre that would be permanently available
around the clock.
Our Approach to Implementation
There are numerous factors to consider when
implementing a new service, including costs, resource
advocacy, specialised training, involvement of key
stakeholders, and patient safety. Local administrative
systems and funding should be considered from the
onset. Our local healthcare system is publicly financed
with revenue from federal, provincial, and territorial
taxation. The Medical Care Plan is a comprehensive
provincial insurance that covers the cost of physician
services for patients, including hospital services.
Physicians receive compensation by negotiating fee
codes and billing Medical Care Plan for insured hospital services when recommended by a medical practitioner.
Different specialists require separate fee codes for each
procedure. New hospital services are approved by the
Regional Health Authority Executive Team. Hospital
departments have separate operating budgets, meaning
that funding for a new neurointerventional radiology
programme would largely need to come from our own
budget. Unfortunately, we encountered many challenges
that introduced significant delays that wasted time,
resources, finances, and training.
Costs
We first considered the cost of implementing a new
service, notably the significant upfront costs to develop
the necessary infrastructure for neurointerventional
procedures. We would need a new biplane angiography
suite (US$2,024,000), a secondary multipurpose
angiography suite for displaced cases (US$748,000),
an inventory of coils and guidewires (US$352,000),
departmental renovations (US$440,000), and salary for
two additional nurses and one technologist.
We estimated that the cost-per-case would be lower if
the service were performed at our centre, as we would
avoid the costs of air transport (US$9303). However, we
recognised that this was not necessarily a money-saving
initiative. Our centre would reallocate the neurosurgery
aneurysm clipping operating room time with other
costly surgeries and available beds would be filled with
other patients. Rather, the major benefit would be not
transferring critically ill patients via air ambulance.
Resource Advocacy
With the support of the Department of Neurosurgery, we approached our hospital’s Vice President of Medicine to
request the necessary resources. There were numerous
meetings in which we emphasised that deferred
implementation of a coiling programme would result in
substandard care. In 2006, our health authority requested
US$3,564,000 in capital governmental funds to meet the
upfront costs for programme implementation. Likely
because of our partnership with the Department of
Neurosurgery (those responsible for managing patient
treatment plans and referrals to our services) and the
recognition that this programme was necessary to meet
the standard of care, a new biplane angiography suite
was quickly approved and installed early in 2008.
Specialised Training
To prepare for programme implementation, we
considered radiologist education. Two of our three interventional neuroradiologists had standard fellowship
training at accredited Canadian institutions, which they
finished in 2001 and 2005. Their fellowship training
included intracranial aneurysm coiling techniques.
The third and most senior neuroradiologist completed
a cerebral coiling sabbatical in early 2008 in tandem
with the installation of the biplane angiography suite.
With 30 cases predicted yearly, our service would have
a sufficient critical volume to maintain cerebral coiling
expertise.
Unexpected Delays due to Late Involvement
of Key Stakeholders
We were hopeful that we could begin coiling in the fall of 2008; however, we encountered further obstacles.
Although casual conversations were conducted, we
erred in not formally involving the Department of
Anaesthesiology from the onset. Getting available
anaesthesiologists was an obstacle, as the angiography
suite was a new location for them to cover. Issues around
fee codes had to be overcome, because the Departments
of Radiology and Anaesthesiology each required
separate fee codes for this new procedure to receive
compensation. Furthermore, given the unscheduled and
urgent nature of many coiling cases, additional overtime
coverage was needed.
Just before implementation of the programme, we were
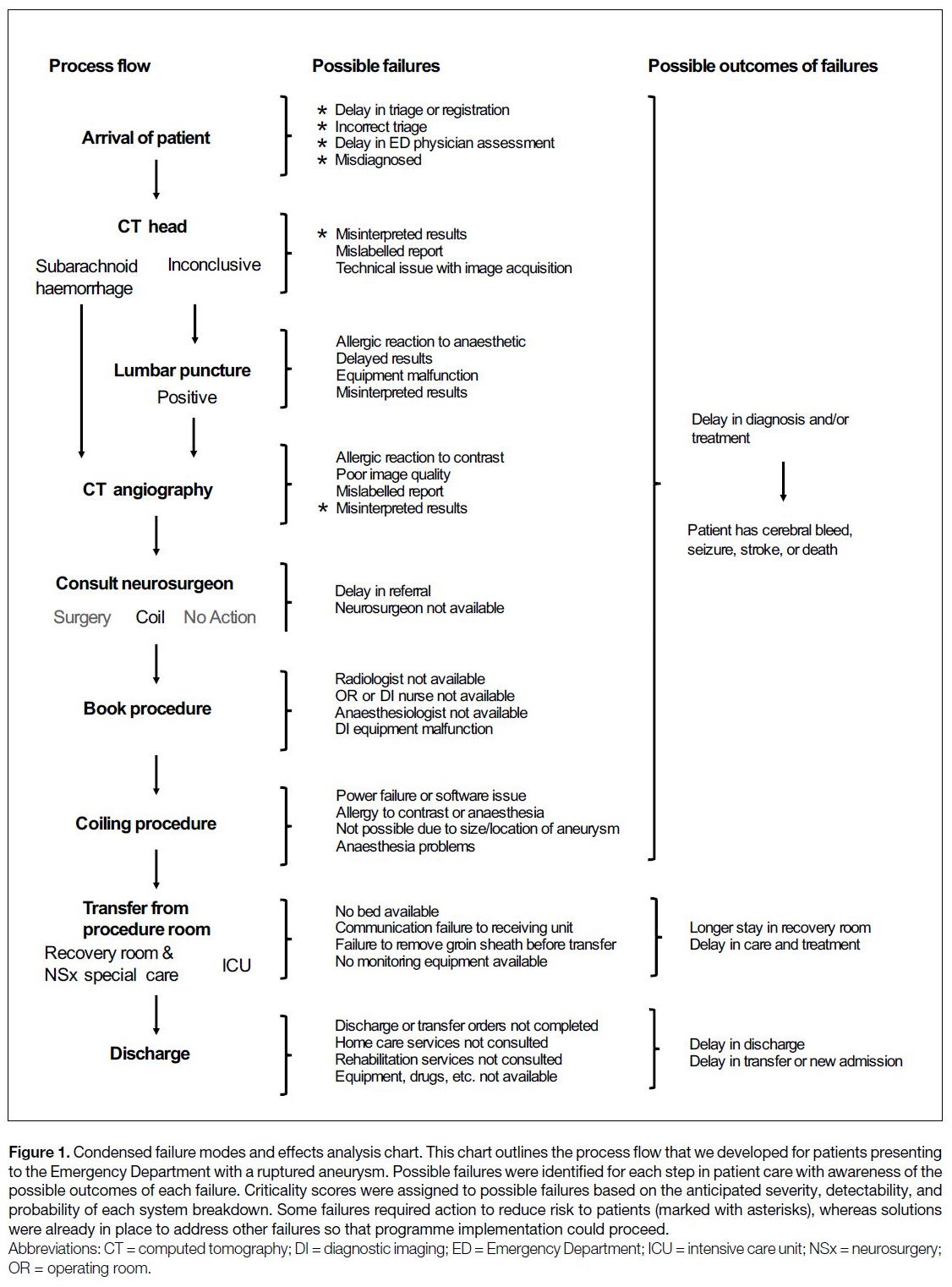
required to complete a failure modes and effects analysis
(FMEA), which is a systematic method of identifying
where and how issues might arise (Figure 1). This
process evaluated all anticipated failures at every step of
patient treatment, from initial examination to discharge
from the hospital after surgery, to calculate patient risk
in all potential scenarios. Our quality assurance team
ranked the severity, detectability, and probability of
potential failures on a scale from 1 to 5 and calculated
the criticality score from the product of these scores. An
unacceptable risk to patients was defined as a score of 5
for severity or a criticality score of >20. An unacceptable
risk score stalled the FMEA and required intervention
by the quality assurance team to adjust procedures and
policies or to provide additional training of staff that may
be involved in the process flow to reduce risks to patients
(Figure 1). After the intervention, the criticality score
was recalculated, and this process was continued until all
potential failures were assigned acceptable severity ranks
and criticality scores to ensure that all considerations
were made. This clarified our workflow and service
model from the onset. For elective cases, neurosurgery
is responsible for initial patient assessment and referral to interventional neuroradiology for aneurysms that can
be clipped or coiled. Interventional neuroradiology will
also assess the patient, discuss the procedure with the
patient, and obtain informed consent. Elective patients
are subsequently assessed in the Outpatient Preoperative
Assessment Clinic by anaesthesiology. All specialists
work together for patient care and can raise concerns
regarding safety and the risk-benefit ratio for the patient.
Neurosurgery is responsible for management after
surgery, with the services again working collaboratively
toward patient discharge. Patients are followed clinically
by neurosurgery and follow-up imaging is completed by
radiology, typically at 3 months after surgery and once
per year thereafter. This arrangement is also followed for
emergency patients, albeit on an accelerated timeline,
with all assessments made in the hospital on an emergent
basis. FMEA enabled us to pre-emptively improve
patient safety by anticipating the logistical issues that
may arise when beginning a new service. A proctor was
present for our initial cases to aid in local programme
implementation. Credentialing was necessary for this
new procedure, which was based on the training of
the interventional neuroradiologists and the volume of
cerebral procedures needed to maintain competence.
Figure 1. Condensed failure modes and effects analysis chart. This chart outlines the process flow that we developed for patients presenting
to the Emergency Department with a ruptured aneurysm. Possible failures were identified for each step in patient care with awareness of the
possible outcomes of each failure. Criticality scores were assigned to possible failures based on the anticipated severity, detectability, and
probability of each system breakdown. Some failures required action to reduce risk to patients (marked with asterisks), whereas solutions
were already in place to address other failures so that programme implementation could proceed.
Costs of Delays
Unfortunately, FMEA is a long process: it was not until March 2013 that all recommendations and actions were
completed. We had returned the initial inventory of coils
and other supplies obtained in 2008. Restocking in 2013
caused additional expense and frustration. Moreover,
delays in programme implementation led to loss of
expertise such that the two of our three interventional
neuroradiologists required costly retraining. One of them
completed additional training over the course of 6 months
at an out-of-province high-volume centre. The other was
unable to initially participate in retraining because of
personal circumstances and is only now in the process
of retraining, which meant the programme relied on the
availability of two interventional neuroradiologists for
years. While our service is offered around the clock, in
rare contingency situations we have still have to transfer
patients by air ambulance to a larger centre depending
on the availability and expertise of the interventional
neuroradiologists.
OUTCOMES OF OUR PROGRAMME IMPLEMENTION
To verify the quality of our newly implemented
neurointerventional programme, we obtained local
ethics approval to initiate a retrospective review of all patients who underwent cerebral coiling from March
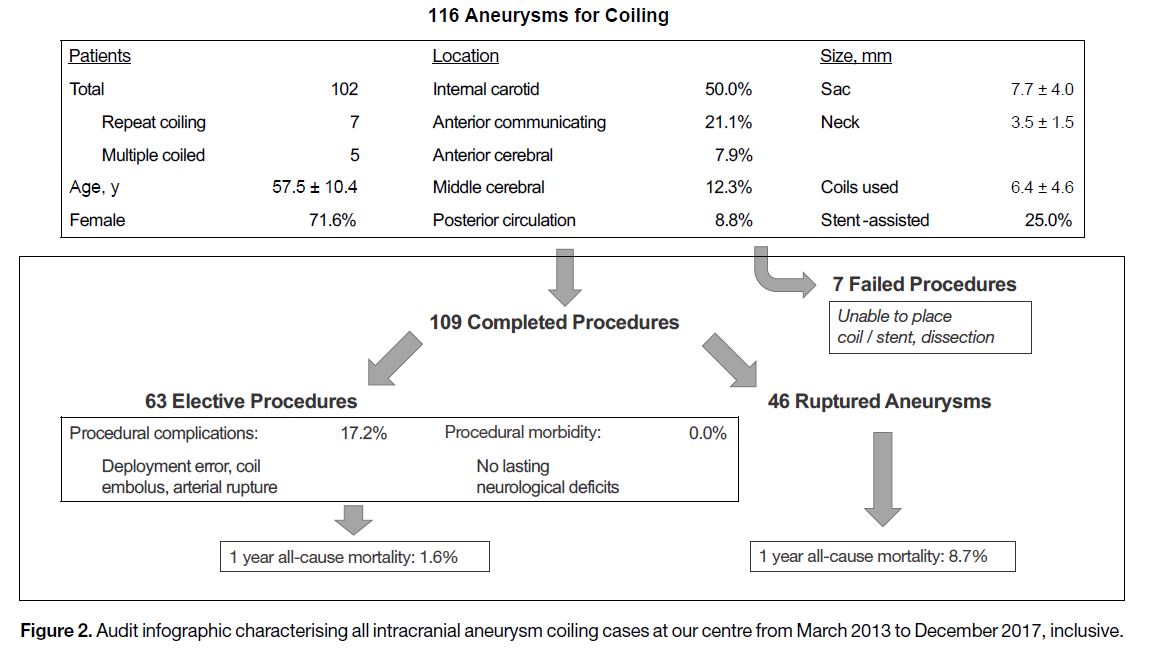
2013 until December 2017 (Figure 2). We found that
116 procedures were attempted on 102 patients, with
seven failures due to coil or stent placement difficulties.
Most aneurysms arose from the internal carotid artery
and over half were coiled electively. Procedural
complications encountered in the elective cases included
deployment error, coil embolisation, and arterial rupture.
As per follow-up neurosurgery examinations, there
were no lasting neurological deficits in patients treated
electively. Among those emergency cases with ruptured
aneurysms, complications were similar, although
transient thrombus formation was also frequently
documented. Patients were followed up at 3 months
after surgery and once per year thereafter with magnetic
resonance angiography to rule out recurrence. The
1-year mortality was 1.6% for elective procedures and
8.7% for ruptured aneurysm repair. This is comparable
to the literature.[7] [8] [9]
Figure 2. Audit infographic characterising all intracranial aneurysm coiling cases at our centre from March 2013 to December 2017, inclusive
CONCLUSION AND FUTURE DIRECTION
The multi-year delay in our programme’s implementation emphasises the need for early involvement of all necessary support disciplines as well as timely quality
and safety assessments. Postponing radiologist
retraining and consumable material consignment instead
of purchase may save costs. Personnel and recruitment
remain ongoing issues for our small centre given that
our programme still relies on the availability of two
interventional neuroradiologists. Other radiologists
in our group absorbed the extra workload, which
underscores the need for additional recruitment when
new services are added. We believe FMEA was integral
for anticipating patient safety concerns and ensuring a
successful programme from the onset.
Ongoing advances in interventional radiology
continually elevate the standard of care, leading to more
advanced procedures being performed at smaller centres.
This requires specialised suites with multidisciplinary
staff, presenting unique challenges to radiology groups
with limited resources and experience in programme
implementation. By outlining the various obstacles that
we faced, we hope other groups can better advocate for
and streamline the introduction of new programmes so
that they do not also experience multi-year delays. We
now find this experience particularly pertinent as we are currently evaluating whether we can offer a sustainable
endovascular thrombectomy programme for ischaemic
stroke.
REFERENCES
1. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of
unruptured intracranial aneurysms, with emphasis on sex, age,
comorbidity, country, and time period: a systematic review and
meta-analysis. Lancet Neurol. 2011;10:626-36. Crossref
2. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635-42. Crossref
3. Lantigua H, Ortega-Gutierrez S, Schmidt JM, Lee K, Badjatia N,
Agarwal S, et al. Subarachnoid hemorrhage: who dies, and why?
Crit Care. 2015;19:309. Crossref
4. Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE,
Lee K, et al. Resuscitation and critical care of poor-grade
subarachnoid hemorrhage. Neurosurgery. 2009;64:397-410. Crossref
5. Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of
saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8-14. Crossref
6. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial
(ISAT) of neurosurgical clipping versus endovascular coiling in
2143 patients with ruptured intracranial aneurysms: a randomised
comparison of effects on survival, dependency, seizures, rebleeding,
subgroups, and aneurysm occlusion. Lancet. 2005;366:809-17. Crossref
7. Batista LL, Mahadevan J, Sachet M, Alvarez H, Rodesch G,
Lasjaunias P. 5-year angiographic and clinical follow-up of
coil-embolised intradural saccular aneurysms. A single center
experience. Interv Neuroradiol. 2002;8:349-66. Crossref
8. Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S,
et al. Endovascular coil occlusion of 1811 intracranial aneurysms:
early angiographic and clinical results. Neurosurgery. 2004;54:268-80. Crossref
9. Bradac GB, Bergui M, Stura G, Fontanella M, Daniele D,
Gozzoli L, et al. Periprocedural morbidity and mortality by
endovascular treatment of cerebral aneurysms with GDC: a
retrospective 12-year experience of a single center. Neurosurg
Rev 2007;30:117-26. Crossref