Computed Tomography Findings in Wunderlich Syndrome
ORIGINAL ARTICLE
Computed Tomography Findings in Wunderlich Syndrome
E Emekli, E Gündoğdu
1 Department of Radiology, Etimesgut Şehit Sait Ertürk State Hospital, Ankara, Turkey
2 Department of Radiology, Eskişehir Osmangazi University, Faculty of Medicine, Eskişehir, Turkey
Correspondence: Dr E Emekli, Department of Radiology, Etimesgut Şehit Sait Ertürk State Hospital, Ankara, Turkey. Email: emreemekli90@gmail.com
Submitted: 2 Nov 2020; Accepted: 11 Feb 2021.
Contributors: Both authors designed the study and acquired the data. EE analysed data and drafted the manuscript. EG critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: Both authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Review Board of Eskişehir Osmangazi University, Faculty of Medicine (Ref: 25403353-050.99-E.38317). The need for patient consent was waived because of the retrospective nature of the study. All patients gave written informed
consent for all treatments and procedures.
Abstract
Background
Wunderlich syndrome (WS) is defined as renal/perirenal haemorrhage in the absence of trauma.
Causative factors include neoplasms, vascular abnormalities, cystic kidney diseases, and coagulation disorders.
Computed tomography (CT) is the diagnostic method of choice in the diagnosis of WS. We aimed to investigate
CT findings, aetiologies, demographic features, and treatment outcomes in a group of patients diagnosed with WS.
Methods
We retrospectively reviewed 113 patients found to have renal-perirenal hematoma on CT between January
2015 and January 2020, and excluded 101 cases with a history of trauma or renal biopsy. The remaining 12 patients
constituted the study group.
Results
Five (41.7%) of the 12 patients included in the study were female and seven (58.3%) were male. The mean
age was 53.2 years (range, 7-81). CT revealed mass lesions in seven (58.3%), a pseudoaneurysm in two (16.7%), and
renal vein thrombosis in one (8.3%) patient. Two of the mass lesions (28.6%) detected on CT were angiomyolipomas,
one (14.3%) was a haemorrhagic cyst in a patient with adult polycystic kidney disease, and four (57.1%) were
solid mass lesions. Three of the four patients with masses were surgically treated. All pseudoaneurysms, the single
inoperable solid mass, and one of the angiomyolipomas were treated angiographically.
Conclusion
WS is an acute urological emergency with multiple possible aetiologies, some of which are more likely
to require surgical management. CT is important in the diagnosis and management of the syndrome to identify haemodynamically unstable cases in need of immediate intervention.
Key Words: Angiomyolipoma; Arteriovenous malformations; Carcinoma, renal cell; Hemorrhage; Kidney/diagnostic imaging
中文摘要
Wunderlich綜合徵的CT表現
E Emekli、E Gündoğdu
背景
Wunderlich綜合徵(WS)定義為無創傷腎或腎週出血。致病因素包括腫瘤、血管異常、囊性腎病和凝血障礙。CT是診斷WS的首選診斷方法。本研究旨在檢視WS患者的CT表現、病因、人口統計學特徵和治療結果。
方法
回顧分析2015年1月至2020年1月期間113例CT發現腎或腎週血腫的患者。排除101例有外傷史或腎活檢史患者後,其餘12名患者構成本研究組。
結果
納入研究的12名患者中,5名(41.7%)為女性,7名(58.3%)為男性。平均年齡53.2歲(範圍:7-81歲)。CT顯示7例患者(58.3%)出現腫塊病變,2例(16.7%)為假性動脈瘤,1例(8.3%)患者出現腎靜脈血栓形成。CT檢測到的腫塊中有2例(28.6%)是血管平滑肌脂肪瘤,1例(14.3%)是成人多囊腎病患者的出血性囊腫,4例(57.1%)是實性腫塊。4例有腫塊的患者中,3例接受手術治療。所有假性動脈瘤、1例無法手術的實性腫塊,和1例血管平滑肌脂肪瘤進行血管造影介入治療。
結論
WS是急性泌尿外科急症,有多種可能病因,部分更可能需要手術治療。CT在WS綜合徵的診斷和臨床處理中很重要,可以識別需要立即干預的血流動力學不穩定病例。
INTRODUCTION
Wunderlich syndrome (WS), first described in 1856
as renal and perirenal haemorrhage in patients without
a history of trauma, is a life-threatening emergency.
Clinically, symptoms are referred to as ‘Lenk’s triad’,
comprising acute flank pain, a palpable abdominal
mass, and haemorrhagic shock. Haematuria is not an
expected finding in patients.[1] Most renal neoplasms
may cause WS, with renal cell carcinoma (RCC) and
angiomyolipoma (AML) reported as the most common
neoplastic causes. Other aetiologies include vascular
abnormalities, including polyarteritis nodosa (PAN),
aneurysms, arteriovenous fistulas, arteriovenous
malformations, renal vein thrombosis (RVT), and
coagulation disorders. Hereditary and acquired cystic
kidney disease and renal infections are also associated
with WS. Although ultrasonography is often the first
method used for diagnosis, computed tomography (CT)
is undertaken to make a definitive diagnosis. Magnetic
resonance imaging (MRI) and angiography are other
imaging methods that can be utilised.[2] [3]
In this paper, we discuss the CT findings, aetiologies, and demographic and medical characteristics of patients
diagnosed with WS, as well as the methods used in their
treatment.
PATIENTS AND METHODS
The study was carried out in accordance with the
principles of the Helsinki Declaration. Between January
2015 and January 2020, 113 patients with perirenal
hematoma were examined using abdominopelvic CT.
Excluded from the study were 101 patients with a history
of trauma or kidney biopsy. The remaining 12 patients
with spontaneous renal/perirenal hematoma constituted
the study group. The demographic characteristics, CT
findings, aetiologies revealed by CT, and the patients’
follow-up and treatment data were recorded. Five
(41.7%) of the 12 patients included in the study were
female and seven (58.3%) were male. The mean age was
53.2 years (range, 7-81). All patients were diagnosed
based on CT findings and clinical and pathological data.
The CT scans were retrospectively evaluated by two
radiologists based on consensus. Both radiologists
diagnosed that all 12 patients had WS. There was no case
of disagreement between the two readers. CT imaging
was performed using a 64-slice (Toshiba Aquilion
64, Tokyo, Japan) or 128-slice (Revolution EVO, GE
Healthcare, Milwaukee [WI], US) multidetector CT
scanner. The subjects were examined in the supine
position with their arms extended above their heads.
All CT examinations were performed using a routine abdominal CT protocol, in which the image data were
acquired from the dome of the diaphragm to the pubic
symphysis after intravenous bolus administration of
iodinated contrast medium with a 65-s delay to visualise
the portovenous phase. Nine patients were first examined
after a 35-s delay to visualise them in cases thought to
be an arterial pathology. The intravenous contrast agent
(1.5 mL/kg; Iopromide 370, Bayer Schering Pharma
AG, Germany or Iohexol 350, GE Healthcare, US)
was administered through an antecubital vein with an
automatic injector at a rate of 3 mL/s.
Commercial software (SPSS Windows version 22.0;
IBM Corp, Armonk [NY], US) was used for statistical
analysis. A normality analysis was performed using the
Shapiro–Wilk test. Descriptive statistics were presented
as mean ± standard deviation for continuous data, and
median and range with percentage values for discrete
data.
RESULTS
Space-occupying lesions were seen on the CT images
of seven patients (58.3%). Pseudoaneurysm formation
was detected in two patients (16.7%) and RVT in one
case (8.3%). For the remaining two patients (16.7%),
the aetiology could not be determined by CT. One
patient had end-stage renal disease and was on a
routine haemodialysis programme. The other patient’s
history included coronary angiography, which had been
performed 2 days earlier. There were findings consistent
with bleeding diathesis in the examination of both
patients, and therefore the aetiology of WS was recorded
as coagulopathy.
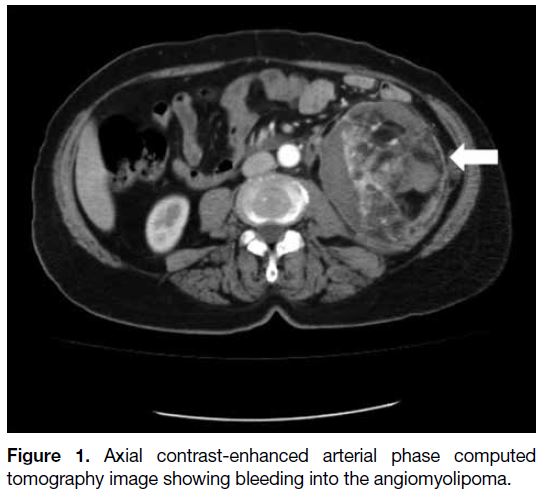
Two of the space-occupying lesions on CT (28.6%)
were AMLs (Figure 1), one (14.3%) was a haemorrhagic cyst in a patient with adult polycystic kidney disease
(APCKD), and four (57.1%) were solid mass lesions. The
demographic and CT data of the patients are summarised
in the Table.
Figure 1. Axial contrast-enhanced arterial phase computed tomography image showing bleeding into the angiomyolipoma
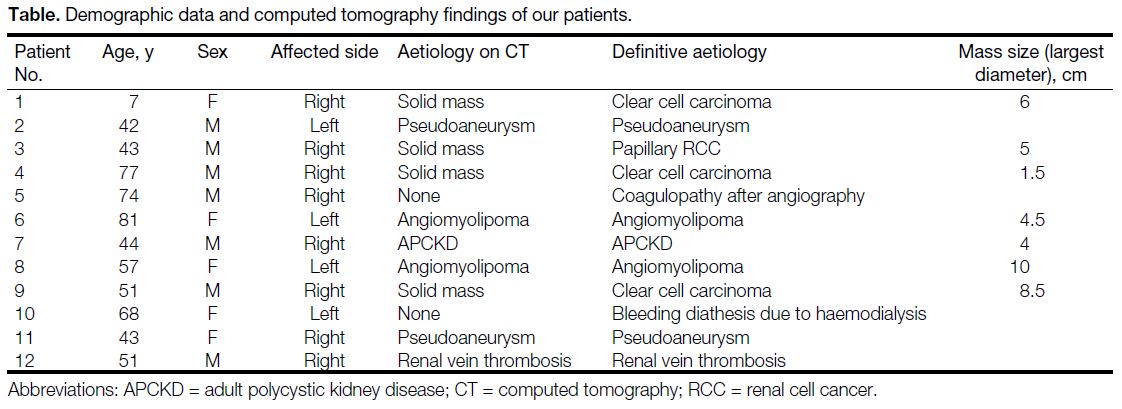
Table. Demographic data and computed tomography findings of our patients
On the CT examinations, hematoma was observed to be
on the right side in eight patients (66.7%) and on the left
side in four patients (33.3%). Two patients had active
extravasation. The cause of WS was AML in one of
these patients and haemodialysis in the other. The widest
diameter in the axial plane of the detected hematomas
ranged from 40 to 161 mm (median, 81.5).
Three of the four patients with masses were surgically treated. Radical nephrectomy was performed in two
patients, and partial nephrectomy in one patient. The
remaining patient was considered inoperable; thus, the
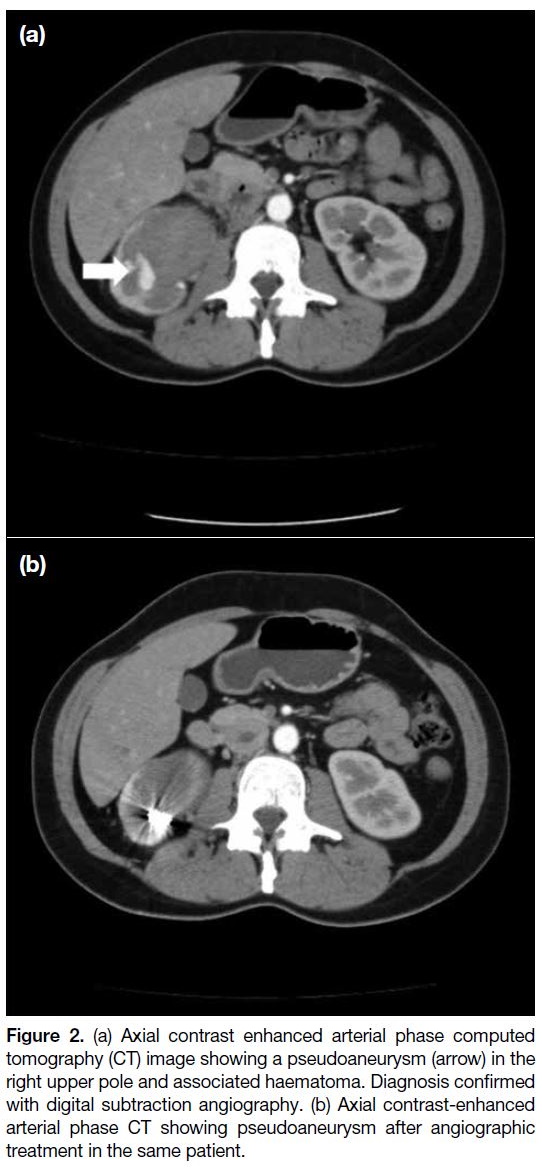
bleeding focus was embolised during angiography. The bleeding focus of two patients with pseudoaneurysms
(Figures 2 and 3) and one of the AML cases were also
angiographically embolised. The patients that developed
haemorrhage due to cysts or after coronary angiography
were discharged once follow-up imaging revealed
improved clinical findings. Three patients died; one with
end-stage kidney disease and two with haemorrhage
due to AML. Thrombectomy was performed during the
angiography on the patient with RVT in the interventional
radiology unit. Recanalisation was achieved and
followed up with anticoagulant therapy.
Figure 2. (a) Axial contrast enhanced arterial phase computed
tomography (CT) image showing a pseudoaneurysm (arrow) in the
right upper pole and associated haematoma. Diagnosis confirmed
with digital subtraction angiography. (b) Axial contrast-enhanced
arterial phase CT showing pseudoaneurysm after angiographic
treatment in the same patient.
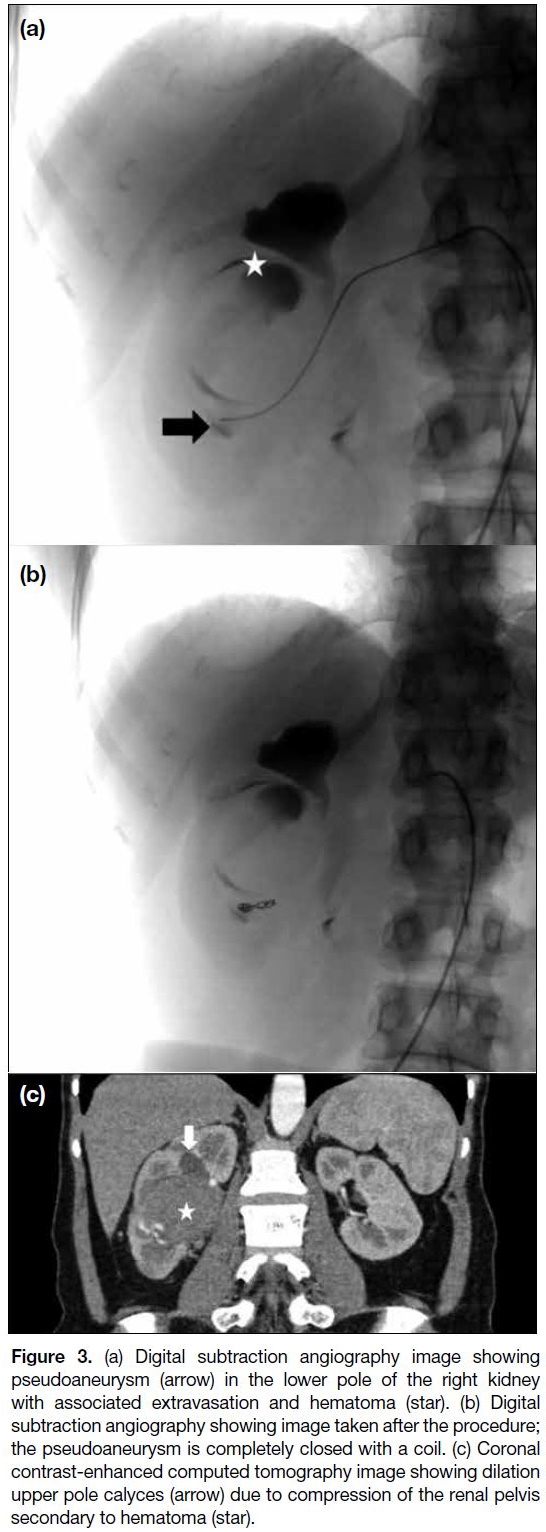
Figure 3. (a) Digital subtraction angiography image showing
pseudoaneurysm (arrow) in the lower pole of the right kidney
with associated extravasation and hematoma (star). (b) Digital
subtraction angiography showing image taken after the procedure;
the pseudoaneurysm is completely closed with a coil. (c) Coronal
contrast-enhanced computed tomography image showing dilation
upper pole calyces (arrow) due to compression of the renal pelvis
secondary to hematoma (star).
All four patients with solid masses detected on CT were diagnosed with RCCs in the pathological evaluation
(three clear cell carcinomas and one papillary cell
carcinoma). According to the radiological, pathological,
and clinical evaluations, the aetiology of WS was
determined to be pseudoaneurysm in two patients, AML
in two patients, RCC in four patients (three clear cell
carcinomas and one papillary cell carcinoma), and one
patient each with a cyst (patient with APCKD), post-angiography
coagulopathy, coagulopathy due to end-stage
kidney failure, and RVT.
DISCUSSION
WS is defined as non-traumatic, spontaneous renal/perirenal haemorrhage, which typically presents with
flank pain, a palpable mass, and haemorrhagic symptoms.
However, it is reported in the literature that only about
20% of patients experience these typical three symptoms
together.4 In a previous study, the rates of abdominal/flank pain, haematuria, and haemorrhagic shock were
67%, 40% and 26.5%, respectively.[4]
In other studies, it has been reported that the male-to-female ratio in WS is approximately 6:5.[3] [5] Similarly, in
our study, WS was more commonly observed among
men, with 58.3% males and 41.7% females. The mean
age of the patients with WS was 53.2 years. In two studies
in the literature, the mean age of the patients has been
reported as 48.1 years and 46.8 years, respectively.[3] [5] These findings are largely consistent with our study.
Different aetiologies can cause this clinical condition. In a meta-analysis conducted by Zhang et al,[3] tumours
(61.5%) were determined to be the most common
aetiology, followed by vascular diseases (17%).
Similarly, in our sample, tumours were the aetiology
in 58.3% of patients (two AMLs, one haemorrhagic
cyst, four RCCs) and vascular causes in 25% (two
pseudoaneurysm and one RVT). In the present study,
RCCs and AMLs ranked first among tumours, which
is in agreement with the literature.[3] AMLs are the most
common benign solid lesions and RCCs are the most
common malign lesions in the general population. WS
is an emergency that requires a rapid diagnosis since it
causes haemorrhagic shock and can lead to death. As
treatment and patient management vary according to
aetiology, determination of the aetiology is paramount.
To this end, urgent diagnostic imaging is essential.
Ultrasonography is often the first imaging method
used in WS. However, due to the known inadequacies
and disadvantages of ultrasonography in evaluating the retroperitoneum, a cross-sectional method, such as MRI
or CT should also be used for a definitive diagnosis.[6]
Both of these modalities have been reported to have
high sensitivity in diagnosis.[7] In addition to conventional
CT, dual-energy CT (DECT) can also be applied to the
diagnosis of WS. In DECT, images are obtained using
the data obtained by scanning the same anatomical region
at two different kVp values (usually 80 and 140 kVp).
The contrast resolution of the data obtained at low kVp
values is high. It is possible to obtain virtual non-contrast
series, iodine perfusion maps, and CT angiography
images by taking advantage of the differences in K
orbital binding energies of iodine and body tissues.[8] [9]
DECT angiography images with bone and calcific
plaque removed may provide better success in showing
active extravasation in WS. It may also contribute to
treatment planning by showing compression of the
renal parenchyma due to haematoma.[9] However, the
necessity of having a double tube or an electronic kVp
changeable tube limits its widespread use. CT should
be the preferred method for the detection of renal/perirenal bleeding and the underlying cause due to its
easier accessibility, shorter examination time during
emergencies, and higher patient compliance and
tolerance.[2] [10] In all of our patients, CT examination was
used for the definitive diagnosis.
AML is the cause of WS in about 30% to 35% of
cases.[3] [11] In our study, AML was detected as the
underlying cause in two patients (16.7%). AML is
considered to originate from perivascular epithelioid
cells located around blood vessels. It contains different
proportions of fat, muscle, and abnormal vascular tissue.
There are two types of AML: the classic triphasic type
and the epithelioid type. The classic type can be sporadic
or accompanied by tuberous sclerosis, whereas the
epithelioid type is less frequently seen and progresses in a
more aggressive manner.[12] [13] Studies report no difference
between the two types in terms of causing WS.[13] On CT,
the classic type of AML is visualised as heterogeneous
mass lesion with high fat content and other components
that are isodense. The epithelioid type may not be
distinguishable from other mass lesions due to its low
fat content.[14] Vascular structures with low elastin content
in AML tend to form aneurysms. This trend increases
as the size of the mass increases. The risk of AML
haemorrhage depends on the size of the lesion and the
diameter of the aneurysm, increasing when the diameter
is >4 cm.[15] In almost all cases of WS secondary to AML,
CT and MRI can be used to identify the underlying mass,
and treatment is performed with catheter embolisation or, if embolisation fails, with surgery. Partial or total
nephrectomy can be performed depending on the size of
the mass.[15] In our study, the mass sizes in the two patients
with AML were 4.5 cm and 10 cm. The bleeding focus
was angiographically embolised in one of these patients
while the other patient died before intervention.
RCC is the second most common cause of WS, reported
as being associated with 26% of WS cases.[3] It is noted
in the literature that WS is only seen in 0.3% to 1.4%
of patients with RCC but due to the high incidence of
RCC, it presents as the most common malignancy
causing WS.[16] Unlike AML, tumour size is not a good
predictor of haemorrhage. When the underlying cause is
RCC, treatment is usually radical nephrectomy.[3] [17] RCC
has three major subtypes: clear cell (70% of all RCCs),
papillary (10%-15%), and chromophobe variants (4%-6%). Clear cell RCC is the most common subtype that
causes WS due to its hypervascularity and rapid growth.
In 60% to 80% of sporadic cases, the von Hippel–Lindau
gene is inactivated, which is considered to activate
various vascular and somatic growth factors and cause
irregular vascularity, thus leading to haemorrhage.[18] In
our study, the most common cause of WS was found to
be RCC, which was seen in four (33.3%) patients. This
rate is largely similar to that in the literature. Similar to
the literature on RCC subtypes, the pathology was clear
cell carcinoma in three patients and papillary carcinoma
in one patient.[2] While three of our patients were treated
surgically, the bleeding focus was angiographically
embolised in the remaining patient because he was not
a surgical candidate.
It is reported that vascular aetiologies are associated with approximately 20% to 30% of WS cases. These include
arterial abnormalities, including PAN, renal artery
aneurysm, and pseudoaneurysm; and venous factors,
such as RVT, renal arteriovenous malformation, and
arteriovenous fistula.[11] PAN is the most common vascular
cause of WS. PAN is a vasculitis inducing multifocal
necrotic areas in medium-size and small arteries,
especially the renal arteries. In patients with PAN, diffuse
enlargement in the kidneys, loss of the corticomedullary
junction, and multiple parenchymal infarcts and
microaneurysms can be seen on CT. The demonstration
of microaneurysms is important in distinguishing PAN
from acute pyelonephritis. Angiography is generally
used in the diagnosis and treatment of PAN when it is
suspected as the cause of WS.[19]
The incidence of pseudoaneurysms and true renal artery aneurysms has been reported as 0.09%.[20] Iatrogenic
causes, inflammation, infection, and vasculitis may play
a role in the aetiology of pseudoaneurysms. CT is the
preferred method for the diagnosis of ruptured aneurysms
because it can reveal massive perinephric haemorrhage
and extravasation foci within the hematoma. Patients with
ruptured aneurysms are usually treated angiographically.[2]
We detected pseudoaneurysms in two of our cases
(16.7%) on CT, which was confirmed by angiography that
was subsequently used for treatment. Compared to the
literature, our rate of pseudoaneurysms was high. RVT
is another important vascular pathology that can cause
WS. RVT may develop secondary to hypercoagulation,
dehydration, and renal masses. In the imaging of patients
with RVT, an enlarged kidney or perinephric oedema in
the renal sinus can be seen. Filling defects in the renal
vein can be demonstrated by contrast-enhanced CT or
MRI. Generally, patients with RVT clinically present
with haematuria, flank pain, and loss of renal function.[2] [21]
RVT was detected in one of our patients (8.3%), who
had impaired renal function, haematuria, and flank pain,
which is consistent with the literature. Studies in the
literature report that WS secondary to RVT is rare.[2] The
high rate of pseudoaneurysms and the presence of RVT
among our patients may be due to our institution being a
tertiary health centre, to which such patients are referred
for angiographic treatment.
Renal cysts often rupture into the pelvicaliceal
system; perinephric rupture is rarely detected. Simple
and haemorrhagic cysts are common causes of WS.
Intracystic haemorrhage is common in APCKD
but secondary perinephric bleeding has rarely been
reported.[22] Among the causes of cyst rupture, intracystic
infection or bleeding are common, and the risk increases
with increasing cyst size.[18] In some cases, large
hematomas may make it difficult to detect the underlying
cause by compressing the ruptured cyst. Follow-up CT
imaging may be required to make a diagnosis. Patients
with cyst rupture are usually treated conservatively,
and antibiotic therapy can be added to treatment if
necessary.[23] In the current study, APCKD was seen in
one of our patients, who did not have any complications
during the follow-up and did not require any surgical or
angiographic procedure.
Coagulation disorders are a heterogeneous group of
diseases. The literature contains studies that are mostly
in the form of case reports indicating coagulation
disorders as a rare cause of WS.[24] [25] In addition, there
are other case reports showing that oral anticoagulant therapy, which is widely used today, can cause WS.[26] It
is known that patients with chronic kidney disease who
undergo haemodialysis have a predisposition to platelet
dysfunction and bleeding secondary to endothelial
abnormalities.[27] [28] Among our cases, WS developed due
to coagulopathy in one patient after coronary angiography
and in another patient that was receiving haemodialysis.
Other causes of WS include infectious diseases, such as acute pyelonephritis, renal abscess, and emphysematous
pyelonephritis. It is reported that infections result in
WS at a rate of approximately 5% to 10%. The risk of
WS is higher in diabetic patients. Parenchymal necrosis
secondary to infection, inflammation-related erosion
of the renal vessels, and intravascular thrombosis are
considered to play a role in its pathogenesis. Idiopathic
WS is responsible for 5% of cases.[2] [3]
CONCLUSION
WS is an acute urological emergency with an aetiologically broad spectrum. The two most common causes of WS are
renal neoplasms and vascular pathologies. Emergency
surgery is required in hemodynamically unstable cases.
CT has an important place in diagnosis, determination of
the underlying aetiology, and management of patients.
Underlying benign pathologies can be successfully
detected using CT, thus avoiding unnecessary
interventional procedures.
REFERENCES
1. Wang BH, Pureza V, Wang H. A tale of Wünderlich syndrome. J Surg Case Rep. 2012;2012:rjs015. Crossref
2. Katabathina VS, Katre R, Prasad SR, Surabhi VR, Shanbhogue AK, Sunnapwar A. Wunderlich syndrome: cross-sectional imaging review. J Comput Assist Tomogr. 2011;35:425-33. Crossref
3. Zhang JQ, Fielding JR, Zou KH. Etiology of spontaneous perirenal
hemorrhage: a meta-analysis. J Urol. 2002;167:1593-6. Crossref
4. Kim JW, Kim JY, Ahn ST, Park TY, Oh MM, Moon DG, et al. Spontaneous perirenal hemorrhage (Wunderlich syndrome): an analysis of 28 cases. Am J Emerg Med. 2019;37:45-7. Crossref
5. Phillips CK, Lepor H. Spontaneous retroperitoneal hemorrhage
caused by segmental arterial mediolysis. Rev Urol. 2006;8:36-40.
6. Expert Panel on Vascular Imaging; Verma N, Steigner ML,
Aghayev A, Azene EM, Chong ST, Desjardins B, et al. ACR
Appropriateness Criteria® suspected retroperitoneal bleed. J Am
Coll Radiol. 2021;18:482-7. Crossref
7. Zagoria RJ, Dyer RB, Assimos DG, Scharling ES, Quinn SF.
Spontaneous perinephric hemorrhage: imaging and management.
J Urol. 1991;145:468-71. Crossref
8. Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG.
Technical principles of dual source CT. Eur J Radiol. 2008;68:362-
8. Crossref
9. Silva AC, Morse BG, Hara AK, Paden RG, Hongo N, Pavlicek W. Dual-energy (spectral) CT: applications in abdominal imaging. Radiographics. 2011;31:1031-46. Crossref
10. Ahn T, Roberts MJ, Navaratna A, Chung E, Wood ST. Wunder-women: systematic review of causes, treatment and outcomes of Wunderlich syndrome during pregnancy. J Clin Urol. 2019;12:134-44. Crossref
11. Cinman AC, Farrer J, Kaufman JJ. Spontaneous perinephric
hemorrhage in a 65-year-old man. J Urol. 1985;133:829-32. Crossref
12. Prasad SR, Sahani DV, Mino-Kenudson M, Narra VR,
Humphrey PA, Menias CO, et al. Neoplasms of the perivascular
epithelioid cell involving the abdomen and the pelvis: cross-sectional
imaging findings. J Comput Assist Tomogr. 2007;31:688-96. Crossref
13. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO,
Prasad SR. Mesenchymal neoplasms of the kidney in adults:
imaging spectrum with radiologic-pathologic correlation.
Radiographics. 2010;30:1525-40. Crossref
14. Wilson MP, Patel D, Katlariwala P, Low G. A review of clinical and MR imaging features of renal lipid-poor angiomyolipomas. Abdom Radiol. 2021;46:2072-8. Crossref
15. Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225:78-82. Crossref
16. Diaz JR, Agriantonis DJ, Aguila J, Calleros JE, Ayyappan AP. Spontaneous perirenal hemorrhage: what radiologists need to know. Emerg Radiol. 2011;18:329-34. Crossref
17. Chang SY, Ma CP, Lee SK. Spontaneous retroperitoneal hemorrhage from kidney causes. Eur Urol. 1988;15:281-4. Crossref
18. Mehdi A, Riazalhosseini Y. Epigenome aberrations: emerging
driving factors of the clear cell renal cell carcinoma. Int J Mol Sci.
2017;16:1774. Crossref
19. Dhanapal V, Ramachandran R, Radhan P, Vivekanandan B,
Jeevanandham B, Jacob P. The many facets of Wunderlich
syndrome: a multidetector computed tomography based review.
Int J Contemp Med Surg Radiol. 2019;4:A88-93. Crossref
20. De Wilde V, Devue K, Vandenbroucke F, Breucq C, De Maeseneer M,
De Mey J. Rupture of renal artery aneurysm into the renal pelvis,
clinically mimicking renal colic: diagnosis with multidetector CT.
Br J Radiol. 2007;80:e262-4. Crossref
21. Kawashima A, Sandler CM, Ernst RD, Tamm EP, Goldman SM, Fishman EK. CT evaluation of renovascular disease. Radiographics. 2000;20:1321-40. Crossref
22. Mabillard H, Srivastava S, Haslam P, Karasek M, Sayer JA. Large
retroperitoneal haemorrhage following cyst rupture in a patient with
autosomal dominant polycystic kidney disease. Case Rep Nephrol.
2017;2017:4653267. Crossref
23. Tarrass F, Benjelloun M. Acute abdomen caused by spontaneous
renal cyst rupture in an ADPKD haemodialysed patient. Nephrology
(Carlton). 2008;13:177-8. Crossref
24. Gomathy SB, Das A, Pandit AK, Srivastava AK. Enoxaparin-induced
Wunderlich syndrome in a young patient with anti-GAD 65-associated opsoclonus and limbic encephalitis: a rare
complication in a rare disease. BMJ Case Rep. 2021;14:e244916. Crossref
25. Gurbani CM, Khor V, Leow JJ, Tay MHW, Chong YL. Wunderlich
syndrome secondary to cyst rupture and concurrent anticoagulation.
Can J Urol. 2020;27:10270-2.
26. Kinnear N, Hennessey DB, Douglass-Molloy H, Jack G. Life-threatening
Wunderlich’s syndrome with concurrent clopidogrel
use. BMJ Case Rep. 2016;9:bcr2016216171. Crossref
27. Xie Y, Yang B, Jiang G, Lu W, Ronco C. Spontaneous perirenal
hemorrhage in hemodialysis patient treated with selective
embolization: A case series and review of the literature. Hemodial
Int. 2018;22:222-7. Crossref
28. Liang CC, Yeh HC, Huang CC, Chang CT. Spontaneous perirenal hepatoma (Wunderlich’s syndrome) in a man on haemodialysis. Nephrology (Carlton). 2010;15:268. Crossref