Risk Factors for Early Mortality in Head and Neck Cancer Patients Undergoing Definitive Chemoradiation
ORIGINAL ARTICLE
Risk Factors for Early Mortality in Head and Neck Cancer Patients Undergoing Definitive Chemoradiation
T Tsui, KM Cheung, JCH Chow, KH Wong
Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr Therese Tsui, Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong. Email: tsui.therese@gmail.com
Submitted: 8 Sep 2021; Accepted: 15 Nov 2021.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Hong Kong Hospital Authority Kowloon Central Cluster/Kowloon East Cluster research ethics committee (Ref: KC/KE-20-0242/ER-1).
Abstract
Introduction
Head and neck cancer (HNC) afflicts >16,000 people in Hong Kong annually. Non-operative treatment
for HNC typically involves radiotherapy (with or without concurrent systemic therapy) and is associated with
significant acute toxicity. Demography, tumour factors, and co-morbidities each influence treatment outcome and
prognosis, but their role in predicting 90-day mortality is less well-known.
Methods
Demographic, clinical, and co-morbidity data of 725 non-metastatic HNC patients (9.4% stage I/II, 90.6%
stage III/IV), who had undergone definitive radiotherapy from 1 January 2016 to 1 March 2020 were collected.
Predictors for 90-day mortality were evaluated by simple and multivariable logistic regression.
Results
We report a 4.6% 90-day mortality rate. Age >60 years(odds ratio [OR] = 3.453, 95% confidence interval [CI] =
1.195-9.928; p = 0.022), Eastern Cooperative Oncology Group performance status (OR = 2.184, 95% CI =
1.071-4.454; p = 0.032) and pre-treatment haemoglobin level (OR = 0.764, 95% CI = 0.596-0.979; p = 0.034)
were significant predictors of 90-day mortality on multivariable analysis. Of the eight co-morbidity scores studied,
the Adult Comorbidity Evaluation–27 (ACE-27) [OR = 2.177, 95% CI = 1.397-3.393; p = 0.001] and the Taipei
Medical University–concurrent chemoradiotherapy Mortality Predictor Score (TMU-CCRT ) [OR = 1.501,
95% CI = 1.134-1.986; p = 0.004) were the most significant predictors of 90-day mortality.
Conclusion
Both clinical factors and co-morbidities predict early mortality in HNC patients. ACE-27 and TMU-CCRT
are appropriate for co-morbidity assessment in relation to early mortality. Further studies to develop prospective
models that identify accurately patients at risk of early mortality during treatment are necessary.
Key Words: Comorbidity; Drug therapy; Head and neck neoplasms; Mortality; Prognosis
中文摘要
接受根治性放化療的頭頸癌患者早期死亡的危險因素
徐譽文、張嘉文、周重行、黃錦洪
引言
香港每年有超過16,000人罹患頭頸癌。頭頸癌的非手術治療通常包括放射治療(聯合或不聯合全身性治療),與顯著急性毒性相關。人口統計學、腫瘤和共病症都會影響治療結果和預後,但它們在預測90天死亡率的作用則鮮為人知。
方法
收集2016年1月1日至2020年3月1日期間接受根治性放療的725例非轉移性頭頸癌患者(I/II期佔9.4%、III/IV期佔90.6%)的人口統計學、臨床和共病症數據。通過簡易及多變量邏輯迴歸評估90天死亡率的預測因子。
結果
90天死亡率為4.6%。60歲以上(優勢比3.453,95%置信區間 = 1.195-9.928;p = 0.022)、ECOG表現狀態(優勢比2.184,95%置信區間 = 1.071-4.454;p = 0.032)和治療前血紅蛋白水平(優勢比0.764,95%置信區間 = 0 596-0.979; p = 0.034)是多變量分析中90天死亡率的重要預測因子。在研究的八種共病症評分中,成人共病症評估27量表(ACE-27;優勢比2.177,95%置信區間 = 1.397-3.393; p = 0.001)及臺北醫學大學同步放化療死亡率預測評分(TMU-CCRT;優勢比1.501,95%置信區間 = 1.134-1.986;p = 0.004)是90天死亡率的最重要預測因子。
結論
臨床因素和共病症均可預測頭頸癌患者的早期死亡率。ACE-27和TMU-CCRT適用於與早期死亡率相關的共病症評估。需要進一步研究發展更全面模式以準確識別治療期間有早期死亡風險的患者。
INTRODUCTION
Radiotherapy and chemotherapy are two of the
mainstays of treatment for head and neck cancer
(HNC).[1] Radiotherapy of HNC may cause mucositis,
dermatitis, or dysphagia, whereas addition of
concomitant chemotherapy increases the risk of
myelosuppression, nausea and vomiting, neurotoxicities,
and nephrotoxicities. Although these typically improve
within 6 months after treatment in most patients,[2]
some patients succumb to these toxicities shortly after
treatment.[3] [4] [5] The United Kingdom introduced a 5%
90-day mortality rate following radical radiotherapy in
HNC as a quality metric.[6] [7]
Treatment outcomes in HNC are influenced by a
range of patient and disease factors. Co-morbidity is
a known independent prognosticator for survival in
HNC.[8] [9] There are several co-morbidity scores available
with known prognostic significance in HNC, some of
which are suitable for registry-based and retrospective
assessment.[8] [10]
The most commonly utilised co-morbidity indices are
the Charlson Comorbidity Index (CCI)[11] and the Adult Comorbidity Evaluation–27 (ACE-27).[12] These have
both been validated in HNC patients.[10] [13] An ACCI
(age-adjusted Charlson Comorbidity Index) is another
established co-morbidity score derived from the CCI and
assigns 1 additional point per decade over 40 years of
age up to 4 points.[14] Although validated in HNC,[15] [16] it is
less widely used than CCI and ACE-27.
There are several assessment tools designed specifically
for HNC patients. The HN-CCI (head and neck
comorbidity index score)[17] and the HNCA (head and
neck cancer index)[18] are both adapted from the CCI.
The WUHNCI (Washington University Head and
Neck Comorbidity Index) contains seven weighted
conditions. It was shown to improve prognostication
over a multivariable regression model comprising
age, sex, race, symptom stage, and TNM stage.[19] The
Simplified Comorbidity Score (SCS) includes seven
co-morbid conditions and smoking status.[20] It was
originally developed for lung cancer patients, but
Göllnitz et al[21] demonstrated its prognostic value in
HNC along with other co-morbidity scores. The Taipei
Medical University–concurrent chemoradiotherapy
(TMU–CCRT) Mortality Predictor Score is the only co-morbidity score developed for prediction of 90-day
mortality post radical chemoradiotherapy in locally
advanced HNC patients.[3]
We sought to assess the 90-day mortality rate after
radical radiotherapy, with or without concurrent systemic
treatment, to identify significant risk factors for early
mortality and to assess the predictive value of different
co-morbidity scores.
METHODS
Patient Cohort
All 1649 consecutive patients treated with radiotherapy
for HNC from 1 January 2016 to 1 March 2020 at a
single tertiary cancer centre were screened for eligibility.
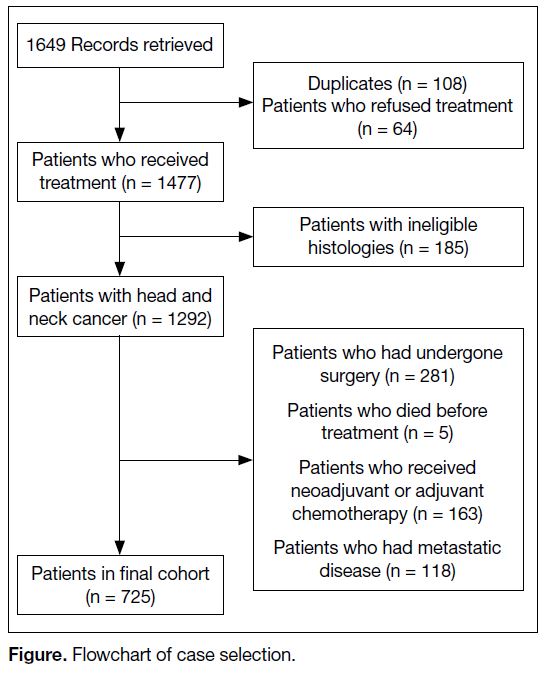
The Figure shows the flowchart for screening. Patients
who were planned for radiotherapy with curative
intent, with or without systemic treatment, as primary
treatment for non-metastatic primary HNC from all sites
and stages were included. To eliminate the effect of
postoperative recovery and chemotherapy before or after
radiotherapy, those who had surgery, and those who
received neoadjuvant or adjuvant chemotherapy with
primary treatment, were excluded. We also excluded
patients who received a planned radiation dose of
<66 Gy to the planning target volume. Patients who were diagnosed with lymphoma, skin malignancies, sarcomas
or thyroid malignancies were excluded. All 725 patients
included in our analysis had received at least 6 months of
follow-up post completion of primary treatment at time
of analysis.
Figure. Flowchart of case selection
Treatment Technique
Radiotherapy to the head and neck region was
administered via intensity-modulated radiotherapy,
volumetric mediated arc therapy, or in the case of
nasopharyngeal cancers (NPC), TomoTherapy.
Radiotherapy doses up to 70-72 Gy in 33 fractions for
NPC and up to 70 Gy in 35 fractions for squamous
HNC were prescribed. Concurrent chemoradiotherapy
regimens included weekly cisplatin 40 mg/m2; cisplatin
100 mg/m2 given once every 3 weeks; or cetuximab
400 mg/m2 1 week before radiotherapy and 250 mg/m2
in subsequent weekly doses until completion of
radiotherapy.
Data Extraction and Calculation of Co-morbidity
Scores
In our study, early mortality was defined as 135 days from start of radiotherapy as a surrogate for 90-day mortality
post radical radiotherapy, assuming a maximum planned
overall treatment time of 45 days.[4]
Manual review and data extraction were performed for
each patient. Demographic information, including age,
sex, body weight and height, smoking history, and alcohol
use were extracted. Eastern Cooperative Oncology
Group (ECOG) performance status was documented.
Tumour site, histology and staging, the results of baseline
swallowing assessment, treatment details including
treatment technique, dose, and concurrent systemic
therapy were logged. Disruptions or unplanned events
warranting medical attention during treatment, including
unplanned feeding tube insertion, admission, or need for
adaptive replanning of radiotherapy were recorded. Start
and end dates of radiotherapy, last date of follow-up, and
date and cause of death were recorded.
Baseline laboratory values including haemoglobin,
white cell count, platelet count, albumin, creatinine,
calcium, and lactate dehydrogenase were retrieved.
Symptom score at presentation, scored as the number of
items present from the following list: dysphagia, otalgia,
weight loss and presence of neck mass, was calculated.[22]
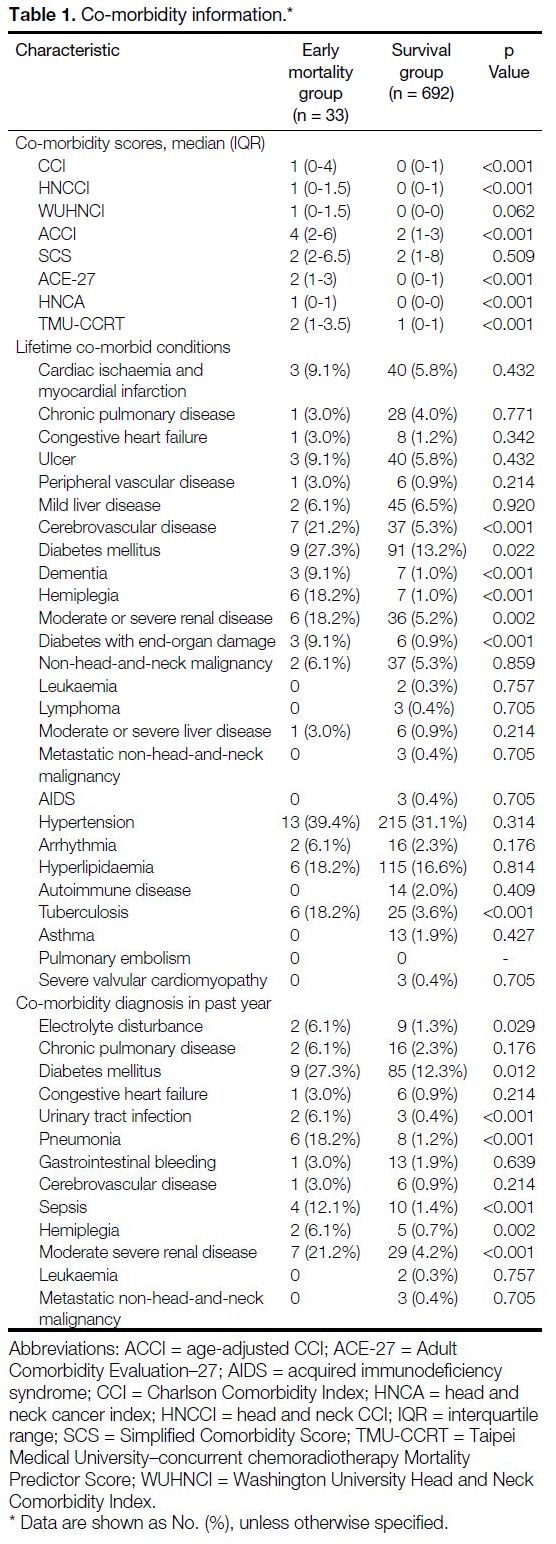
Patient records were reviewed for co-morbidities comprising seven of the eight co-morbidity scores mentioned — CCI, ACCI, HN-CCI, SCS, WUHNCI,
HNCA and TMU-CCRT. The calculation of ACE-27
was assessed directly from electronic medical records.
The full list of co-morbidities assessed is shown in
Table 1.
Table 1. Co-morbidity information
Statistical Analysis
The full cohort was analysed for demographic
information. Univariate analysis was performed using
the Chi-square test for categorical data and the Mann-Whitney U test for continuous or ordinal data to compare
the 90-day mortality and survival groups. Multivariable
logistic regression was performed to evaluate the
effect of different variables on 90-day mortality after
chemoradiotherapy, controlling for other covariates. A p
value <0.05 was taken as significant.
RESULTS
In total, among 1649 consecutive patients, 1292 were
treated with radiotherapy for HNC during the study
period from 1 January 2016 to 1 March 2020 (Figure).
After exclusions for prior surgery (n = 281), neoadjuvant
or adjuvant chemotherapy (n = 163), metastatic disease
(n = 118), or death before treatment (n = 5), finally
725 patients were included in our cohort and received
≥6 months of follow-up after completion of radiotherapy
at time of analysis (Figure).
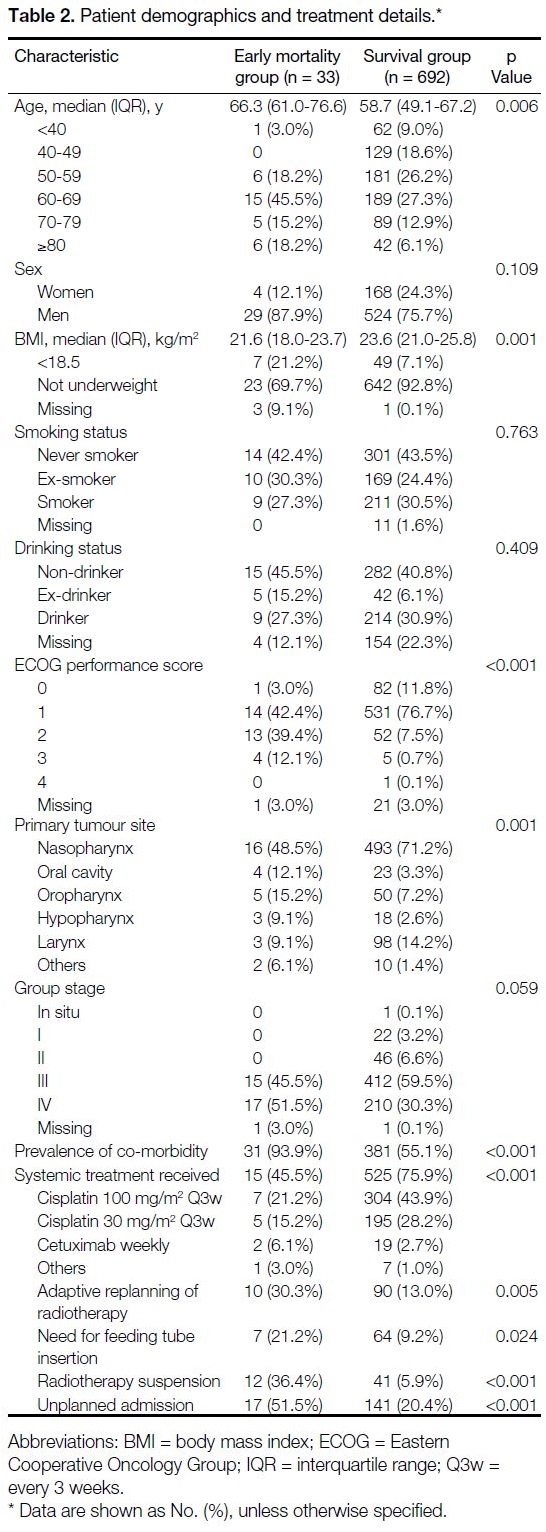
The median age at diagnosis was 59 years. A total of
19.6% were aged >70 years, and 76.3% were male.
Lifetime smokers and drinkers made up 55% and 37%
of cases, respectively. The most common tumour site
was nasopharynx (70.2%), followed by larynx (13.9%),
oropharynx (7.6%), oral cavity (3.7%), hypopharynx
(2.9%), and other sites (1.6%). Locally advanced disease
(stage III/IV) made up 90.6% of cases. A total of 74.1%
received chemotherapy and radiotherapy. The median
follow-up was 23.1 months as of 30 November 2020
(Table 2).
Table 2. Patient demographics and treatment details
In all, 33 out of 725 patients (4.6%) did not survive
beyond 90 days from the end of treatment. Of these, 14
patients (42.4%) died due to pneumonia or sepsis, nine
(27.3%) developed sudden cardiac arrest. Four died
due to distant relapse, and five died due to other causes
including acute kidney injury, pneumoperitoneum, or
deterioration after early termination of treatment. Cause
of death was not available for one patient.
Poorer radiation tolerance was noted in the 90-day
mortality group, with significantly higher rates of adaptive radiotherapy replanning (30.3% vs. 13.0%,
p = 0.005), feeding tube insertion during treatment
(21.2% vs. 9.2%, p = 0.024), radiotherapy suspension
(36.4% vs. 5.9%, p < 0.001) and unplanned admissions
(51.5% vs. 5.9%, p < 0.001).
A total of 57% of cases had at least one co-morbid
condition (Table 1). The 90-day mortality group had
higher median scores across all of the co-morbidity
indices except SCS. A history of stroke (21.2% vs. 5.3%,
p < 0.001), diabetes (27.3% vs. 13.2%, p = 0.022) or
diabetes with complications (9.1% vs. 0.9%, p < 0.001),
dementia (9.1% vs. 1.0%, p < 0.001), hemiplegia (18.2%
vs. 1.0%, p < 0.001) and moderate-to-severe renal
disease (18.2% vs. 5.2%, p = 0.002) were more common
in the 90-day mortality group. Within 1 year of diagnosis
of HNC, electrolyte disturbance (6.1% vs. 1.3%, p = 0.029), sepsis (12.1% vs. 1.4, p < 0.001), pneumonia
(18.2% vs. 1.2%, p < 0.001), and urinary tract infections
(6.1% vs. 0.4%, p < 0.001) were more common in the
90-day mortality group.
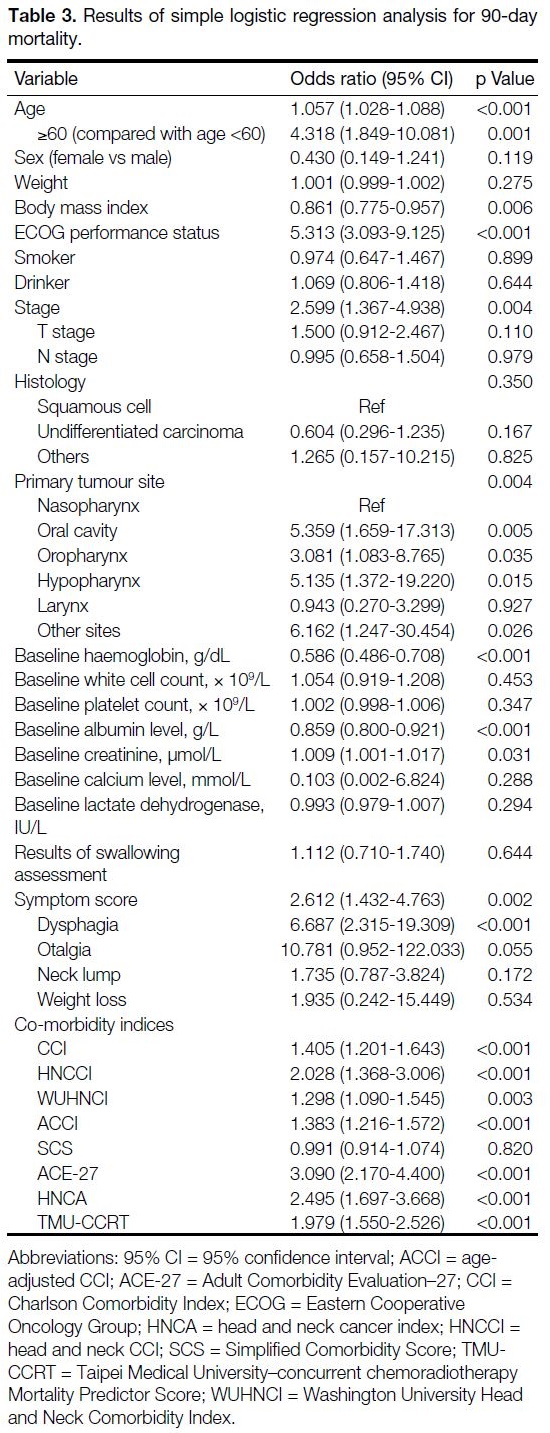
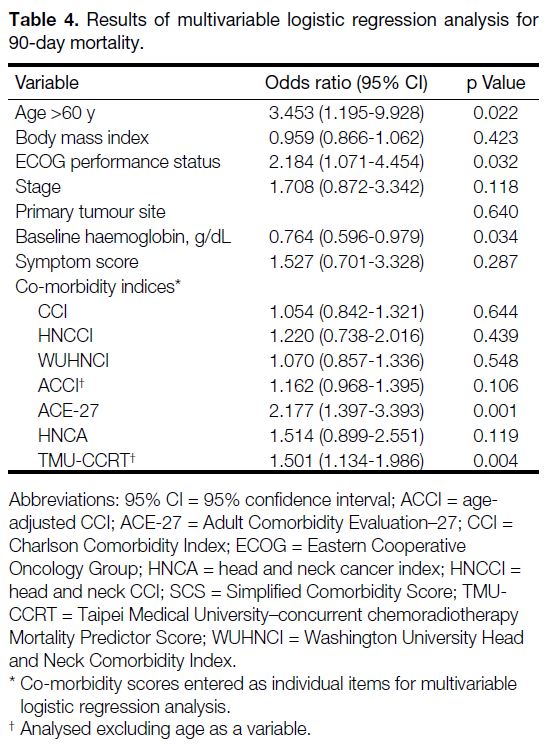
Results of simple logistic regression analysis are shown in Table 3. Multivariable logistic regression analysis
(Table 4) identified age >60, ECOG performance
status, and pre-treatment haemoglobin as significant
independent predictors of 90-day mortality. Of the co-morbidity
indices investigated, when controlled for
clinical parameters, only ACE-27 and TMU-CCRT
remained significant on multivariable regression
analysis.
Table 3. Results of simple logistic regression analysis for 90-day mortality
Table 4. Results of multivariable logistic regression analysis for 90-day mortality
DISCUSSION
Our study found a 4.6% 90-day post-treatment mortality rate, which was within the 5% cut-off per the National
Institute of Clinical Excellence and the Scottish Cancer
Taskforce recommendations.[6] [7] Other real-world cohorts
have reported early mortality rates of 5% to 18%.[3] [23] [24]
Historical randomised controlled trials, which reported
early mortality rates of 1% to 5%, defined the early
mortality period differently, ranging from 30 days from
end of treatment to 90 days from the start of treatment.[25] [26] [27]
As in our cases, the addition of chemotherapy in these
studies was associated with a lower early mortality
rate, although this difference is likely attributable to
confounding due to selection of fitter patients for more
intensive treatment.[25] [26] [27]
Early mortality in NPC is less studied compared to locally
advanced squamous cell carcinomas. Despite sharing
similar radiotherapy doses, organs at risk and systemic treatment regimens, NPC, having a different aetiology
and clinical course, has been purposely excluded
from HNC studies to facilitate long-term prognostic
analysis.[3] [4] [5] In our study, we included NPC, which made
up 69.9% of our cases, due to similarities in primary
treatment between NPC and locally advanced HNCs.
A similar acute toxicity profile is expected. Our results
confirm that the risk of early mortality, after adjusting for
different clinical parameters, is not significantly different
in patients with squamous cell HNC of other primary
sites.[4]
Multivariable logistic regression analysis identified age,
ECOG performance status, and haemoglobin levels as
significant predictors of early mortality. The effect of
age and performance status on prognosis in HNC is well-known.[28] Elderly and frail patients were traditionally
excluded from HNC trials,[25] [26] [27] although exclusion based
on age alone is no longer recommended on the basis of
evidence showing no significant differences in objective
acute toxicity measurements or late toxicity across
different age cut-offs for radical radiotherapy.[29] In patients
aged >70 years, however, the addition of chemotherapy yielded no survival benefit.[1] In our study, we showed that
increasing age remained an important predictor for early
mortality. Performance status is often used as a proxy
for burden of co-morbidity, but other studies have shown
that it provides prognostic information independent of
co-morbidity.[30] [31] The retention of ECOG performance
status with certain co-morbidity indices in multivariable
logistic regression analysis confirms that both have
important implications in predicting early mortality.
In squamous cell HNCs, pre-treatment co-morbidity has
consistently been identified as an important independent
prognosticator. The role of co-morbidity in NPC is
less pronounced, as the Epstein–Barr virus, rather
than alcohol and tobacco exposure, is the dominant
aetiological factor. Despite this, we found ACE-27
and TMU-CCRT to be independent predictors of early
mortality after adjusting for baseline demographic
factors. ACE-27 is widely used for co-morbidity
assessment across different cancers; it has been shown
to predict severe acute toxicity, early mortality, as well
as long-term prognosis in HNC.[4] [10] [32] [33] ACE-27 differs
from other co-morbidity indices covered here in that
it grades overall severity of co-morbidity from 0 (no
co-morbidity) to 3 (severe co-morbidity).[12] It also covers
a wider scope of co-morbid conditions compared with
other co-morbidity indices under review.[10] Currently, it
is the recommended standard for recording co-morbidity
data in the United Kingdom.[34] TMU-CCRT scores
patients for early mortality risk based on stratified age
and six other co-morbid conditions.[3] Our results validate
the role of TMU-CCRT as a predictor of early mortality
in HNC.
Based on median scores in the 90-day mortality and
survival groups, ACE-27 was best able to differentiate
between severity levels of co-morbidity. An ACE-27
score of 2 corresponds to a moderate level of co-morbidity,
while a score of 0 corresponds to no co-morbidity. In
contrast, the median scores for TMU-CCRT in the
90-day mortality and survival groups both fell into the
low-risk category based on the proposed stratification
by Lin et al.[3] This illustrates the need for universally
appropriate cut-offs for clinical use. Our review of
the current literature found that, apart from ACE-27,
different cut-offs have been utilised by different groups
to delineate the co-morbidity burden.
Pre-treatment laboratory values are relatively
less investigated as prognostic markers.[24] These
haematological and biochemical markers may reflect baseline co-morbidity, but not all mechanisms by which
they affect survival or treatment outcomes are well
understood. In our study, we found that haemoglobin
levels below the lower limit of normal was associated
with early mortality. In HNC, anaemia is associated with
higher recurrence rates and poorer overall survival,[21] [35] an
effect attributed to decreased efficacy of treatment due to
tumour hypoxia and decreased radiosensitivity.[36] [37] Other
studies have also demonstrated that early mortality was
more likely in patients with baseline anaemia.[4] After
controlling for other factors, other blood parameters
were not correlated with early mortality in our cases.
Sepsis and pneumonia were the most common causes
of death among patients who died within 90 days after
completing treatment. Radiotherapy to the head and
neck often causes acute toxicities such as mucositis,
dysphagia, and odynophagia, increasing the risk of
aspiration.[38] Eisbruch et al[39] reported aspiration rates up
to 65% within 3 months after radiotherapy, while other
studies have found aspiration pneumonia rates up to
17.6%.[40] This may be exacerbated by concomitant use
of chemotherapy or biologics. Collapse or cardiac events
were the second most common, supporting previous
work that suggested higher risk of cardiovascular events
in cancer patients.[41]
Despite attempts to identify risk factors for early mortality
following radical treatment for HNC, the optimal case
selection criteria for intensive treatment remains elusive.
Substandard treatment is independently associated with
poorer survival after adjusting for other factors such
as performance status, age, and mild co-morbidity,[42]
emphasising the need for comprehensive assessment of
fitness for treatment.
There are several limitations to our study. Our data
were obtained from a single tertiary cancer centre and
consisted of predominantly NPC patients. Analysis from
a population-based database and further validation in
squamous cell HNC population is necessary to confirm
generalisability of our results. Second, information
regarding baseline functional status or socioeconomic
status was not available from retrospective review of
patient records. These are potentially important factors in
predicting tolerance to treatment.[43] [44] Crucially, although
presence of one or more these factors indicates a higher
odds of mortality, this does not definitively identify
patients who will suffer early death within 90 days after
treatment; as such, these factors should not be used to
exclude patients from potentially curative treatment. The best course of action in an individual with multiple risk
factors is not known and the formulation of any treatment
plan requires careful discussion with the patient.
CONCLUSION
Minimising early treatment mortality is important for
optimising outcomes of patients with HNC. The 90-day
mortality rate after radical radiotherapy, with or without
concurrent systemic treatment, in our cohort was 4.6%.
Age, pre-treatment Hb, ECOG performance status,
and two co-morbidity indices: ACE-27 and TMU-CCRT,
were found to be independent predictors of
early mortality. Development of a more comprehensive
prediction model from these factors may help with case
selection of patients for intensive HNC treatment.
REFERENCES
1. Pignon JP, le Maître A, Maillard E, Bourhis J, MACH-NC
Collaborative Group. Meta-analysis of chemotherapy in head and
neck cancer (MACH-NC): an update on 93 randomised trials and
17,346 patients. Radiother Oncol. 2009;92:4-14. Crossref
2. Rathod S, Gupta T, Ghosh-Laskar S, Murthy V, Budrukkar A,
Agarwal J. Quality-of-life (QOL) outcomes in patients with
head and neck squamous cell carcinoma (HNSCC) treated with
intensity-modulated radiation therapy (IMRT) compared to three-dimensional
conformal radiotherapy (3D-CRT): evidence from a
prospective randomized study. Oral Oncol. 2013;49:634-42. Crossref
3. Lin KC, Chen TM, Yuan KS, Wu AT, Wu SY. Assessment of
predictive scoring system for 90-day mortality among patients
with locally advanced head and neck squamous cell carcinoma
who have completed concurrent chemoradiotherapy. JAMA Netw
Open. 2020;3:e1920671. Crossref
4. Dixon L, Garcez K, Lee LW, Sykes A, Slevin N, Thomson D.
Ninety day mortality after radical radiotherapy for head and neck
cancer. Clin Oncol (R Coll Radiol). 2017;29:835-40. Crossref
5. Hamilton SN, Tran E, Berthelet E, Wu J, Olson R. Early (90-day)
mortality after radical radiotherapy for head and neck squamous cell
carcinoma: a population-based analysis. Head Neck. 2018;40:2432-40. Crossref
6. National Institute for Clinical Excellence. Improving outcomes in
head and neck cancers. Available from: https://www.nice.org.uk/guidance/csg6. Accessed 7 Jun 2022.
7. Scottish Cancer Taskforce. Head and neck cancer clinical quality
performance indicators. Patients diagnosed during April 2014 to
March 2015. Available from: https://www.isdscotland.org/Health-Topics/Quality-Indicators/Publication.... Accessed 7 Jun 2022.
8. Bøje CR. Impact of comorbidity on treatment outcome in head and
neck squamous cell carcinoma — a systematic review. Radiother
Oncol. 2014;110:81-90. Crossref
9. Paleri V, Wight RG, Silver CE, Haigentz M Jr, Takes RP,
Bradley PJ, et al. Comorbidity in head and neck cancer: a
critical appraisal and recommendations for practice. Oral Oncol.
2010;46:712-9. Crossref
10. Rogers SN, Aziz A, Lowe D, Husband DJ. Feasibility study of
the retrospective use of the Adult Comorbidity Evaluation index
(ACE-27) in patients with cancer of the head and neck who had
radiotherapy. Br J Oral Maxillofac Surg. 2006;44:283-8. Crossref
11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies:
development and validation. J Chronic Dis. 1987;40:373-83. Crossref
12. Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593-602. Crossref
13. Singh B, Bhaya M, Stern J, Roland JT, Zimbler M, Rosenfeld RM,
et al. Validation of the Charlson comorbidity index in patients with
head and neck cancer: a multi-institutional study. Laryngoscope.
1997;107:1469-75. Crossref
14. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245-51. Crossref
15. Tanaka H, Takenaka Y, Nakahara S, Hanamoto A, Fukusumi T,
Michiba T, et al. Age-adjusted Charlson comorbidity index
as a prognostic factor of hypopharyngeal cancer treated with
chemoradiation therapy. Acta Otolaryngol. 2017;137:668-73. Crossref
16. Yang CC, Chen PC, Hsu CW, Chang SL, Lee CC. Validity of the
age-adjusted Charlson comorbidity index on clinical outcomes
for patients with nasopharyngeal cancer post radiation treatment:
a 5-year nationwide cohort study. PLoS One. 2015;10:e0117323. Crossref
17. Bøje CR, Dalton SO, Primdahl H, Kristensen CA, Andersen E,
Johansen J, et al. Evaluation of comorbidity in 9388 head and
neck cancer patients: a national cohort study from the DAHANCA
database. Radiother Oncol. 2014;110:91-7. Crossref
18. Reid BC, Alberg AJ, Klassen AC, Rozier RG, Garcia I, Winn DM,
et al. A comparison of three comorbidity indexes in a head and neck
cancer population. Oral Oncol. 2002;38:187-94. Crossref
19. Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of
a new head and neck cancer-specific comorbidity index. Arch
Otolaryngol Head Neck Surg. 2002;128:1172-9. Crossref
20. Colinet B, Jacot W, Bertrand D, Lacombe S, Bozonnat MC,
Daurès JP, et al. A new simplified comorbidity score as a prognostic
factor in non-small-cell lung cancer patients: description and
comparison with the Charlson’s index. Br J Cancer. 2005;93:1098-105. Crossref
21. Göllnitz I, Inhestern J, Wendt TG, Buentzel J, Esser D, Böger D,
et al. Role of comorbidity on outcome of head and neck cancer:
a population-based study in Thuringia, Germany. Cancer Med.
2016;5:3260-71. Crossref
22. Pugliano FA, Piccirillo JF, Zequeira MR, Fredrickson JM,
Perez CA, Simpson JR. Symptoms as an index of biologic
behavior in head and neck cancer. Otolaryngol Head Neck Surg.
1999;120:380-6. Crossref
23. Capuano G, Grosso A, Gentile PC, Battista M, Bianciardi F, Di Palma A, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck. 2008;30:503-8. Crossref
24. Schlumpf M, Fischer C, Naehrig D, Rochlitz C, Buess M. Results
of concurrent radio-chemotherapy for the treatment of head and
neck squamous cell carcinoma in everyday clinical practice with
special reference to early mortality. BMC Cancer. 2013;13:610. Crossref
25. Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF,
et al. An intergroup phase III comparison of standard radiation
therapy and two schedules of concurrent chemoradiotherapy in
patients with unresectable squamous cell head and neck cancer. J
Clin Oncol. 2003;21:92-8. Crossref
26. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH,
Saxman SB, et al. Postoperative concurrent radiotherapy and
chemotherapy for high-risk squamous-cell carcinoma of the head
and neck. N Engl J Med. 2004;350:1937-44. Crossref
27. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R,
Morrison W, et al. Concurrent chemotherapy and radiotherapy for
organ preservation in advanced laryngeal cancer. N Engl J Med.
2003;349:2091-8. Crossref
28. Sze HC, Ng WT, Chan OS, Shum TC, Chan LL, Lee AW. Radical radiotherapy for nasopharyngeal carcinoma in elderly patients: the importance of co-morbidity assessment. Oral Oncol. 2012;48:162-7. Crossref
29. Pignon T, Horiot JC, Van den Bogaert W, Van Glabbeke M,
Scalliet P. No age limit for radical radiotherapy in head and neck
tumours. Eur J Cancer. 1996;32A:2075-81. Crossref
30. Wang JR, Habbous S, Espin-Garcia O, Chen D, Huang SH,
Simpson C, et al. Comorbidity and performance status as
independent prognostic factors in patients with head and neck
squamous cell carcinoma. Head Neck. 2016;38:736-42. Crossref
31. Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582-7. Crossref
32. Monteiro AR, Garcia AR, Pereira TC, Macedo F, Soares RF,
Pereira K, et al. ACE-27 as a prognostic tool of severe acute
toxicities in patients with head and neck cancer treated with
chemoradiotherapy: a real-world, prospective, observational study.
Support Care Cancer. 2021;29:1863-71. Crossref
33. Sanabria A, Carvalho AL, Vartanian JG, Magrin J, Ikeda MK,
Kowalski LP. Validation of the Washington University Head
and Neck Comorbidity Index in a cohort of older patients. Arch
Otolaryngol Head Neck Surg. 2008;134:603-7. Crossref
34. Robson A, Sturman J, Williamson P, Conboy P, Penney S, Wood H.
Pre-treatment clinical assessment in head and neck cancer: United
Kingdom National Multidisciplinary Guidelines. J Laryngol Otol.
2016;130:S13-22. Crossref
35. Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, et al.
Anemia is associated with decreased survival and increased
locoregional failure in patients with locally advanced head and
neck carcinoma: a secondary analysis of RTOG 85-27. Int J Radiat
Oncol Biol Phys. 1998;42:1069-75. Crossref
36. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent
prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214-21. Crossref
37. Hoff CM. Importance of hemoglobin concentration and its
modification for the outcome of head and neck cancer patients
treated with radiotherapy. Acta Oncol. 2012;51:419-32. Crossref
38. Russi EG, Corvò R, Merlotti A, Alterio D, Franco P, Pergolizzi S, et al.
Swallowing dysfunction in head and neck cancer patients treated
by radiotherapy: review and recommendations of the supportive
task group of the Italian Association of Radiation Oncology. Cancer
Treat Rev. 2012;38:1033-49. Crossref
39. Eisbruch A, Lyden T, Bradford CR, Dawson LA, Haxer MJ,
Miller AE, et al. Objective assessment of swallowing dysfunction
and aspiration after radiation concurrent with chemotherapy for
head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23-8. Crossref
40. Kawashita Y, Morimoto S, Tashiro K, Soutome S, Yoshimatsu M,
Nakao N, et al. Risk factors associated with the development of
aspiration pneumonia in patients receiving radiotherapy for head
and neck cancer: retrospective study. Head Neck. 2020;42:2571-80. Crossref
41. Fang F, Fall K, Mittleman MA, Sparén P, Ye W, Adami HO, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310-8. Crossref
42. Sanabria A, Carvalho AL, Vartanian JG, Magrin J, Ikeda MK,
Kowalski LP. Factors that influence treatment decision in older
patients with resectable head and neck cancer. Laryngoscope.
2007;117:835-40. Crossref
43. Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH,
van Munster BC. Frailty screening methods for predicting outcome
of a comprehensive geriatric assessment in elderly patients with
cancer: a systematic review. Lancet Oncol. 2012;13:e437-44. Crossref
44. Gaubatz ME, Bukatko AR, Simpson MC, Polednik KM, Boakye EA,
Varvares MA, et al. Racial and socioeconomic disparities associated
with 90-day mortality among patients with head and neck cancer
in the United States. Oral Oncol. 2019;89:95-101. Crossref