Imaging of Idiopathic Granulomatous Mastitis: a Retrospective Study
ORIGINAL ARTICLE
Imaging of Idiopathic Granulomatous Mastitis: a Retrospective Study
CPY Chien1, CSY Lo2, TPW Lam1
1 Department of Radiology, Queen Mary Hospital, Hong Kong
2 Department of Diagnostic and Interventional Radiology, Hong Kong Sanatorium & Hospital, Hong Kong
Correspondence: Dr CPY Chien, Department of Radiology, Queen Mary Hospital, Hong Kong. Email: chienpyc@gmail.com
Submitted: 12 Mar 2021; Accepted: 2 Aug 2021.
Contributors: All authors designed the study. CPYC and CSYL acquired and analysed the data. CPYC drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This retrospective study was approved by Hong Kong West Cluster Research Ethics Committee and the requirement to obtain informed consent was waived (IRB Ref: UW19-447).
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Declaration: The results of this study were previously presented at the European Congress of Radiology in 2020. Permission to re-use the figures was granted.
Abstract
Introduction
The aim of this study was to review the clinical manifestations, imaging findings, and management of
idiopathic granulomatous mastitis (IGM).
Methods
We retrospectively analysed the clinical and imaging findings of all women diagnosed with IGM at our
tertiary care hospital from July 2012 to June 2019.
Results
Of the 29 women (31 breasts) included, 24 patients were of childbearing age. Nine of them had a history
of breastfeeding within the past year. Twenty-four patients had been misdiagnosed as breast cancer or abscess
initially. Nine patients were documented to have hyperprolactinaemia. Imaging findings were nonspecific, with
the most frequent ultrasound finding of an irregular hypoechoic mass in 17 (54.8%) of 31 breasts, and the most
frequent mammographic finding of a focal asymmetry in six (37.5%) of 16 breasts. Fine needle aspiration biopsy was
diagnostic in four (23.5%) of 17 lesions. All 24 ultrasound-guided core biopsies were diagnostic. Corynebacterium
species was found in four samples (12.9%). 16 patients (55.2%) were treated medically with a combination of steroids
and antibiotics. Drainage was performed in 18 lesions (58.1%). Thirteen patients (44.8%) had a recurrence with a
median follow-up of 21 months
Conclusion
IGM is a rare benign chronic inflammatory breast disease with no specific clinical or imaging features.
Diagnosis should be made by biopsy based on the clinical presentation.
Key Words: Breast; Granulomatous mastitis; Inflammation
中文摘要
特發性肉芽腫性乳腺炎的影像學:回顧性研究
錢珮恩、羅承恩、林培榮
引言
回顧分析特發性肉芽腫性乳腺炎(IGM)的臨床及影像學表現和治療。
方法
回顧性分析2012年7月至2019年6月在本三級醫院診斷為IGM所有女性的臨床和影像學表現。
結果
納入的29名女性(31個乳腺)中,24名患者處於育齡期。其中9人在過去一年內有喂乳史。24名患者最初被誤診為乳腺癌或膿腫。9名患者被認為有高泌乳素症。影像學表現無特異性。最常見的超聲發現為不規則低迴聲腫塊,見於31個乳腺中17個(54.8%)。最常見的乳腺X線檢查發現為局灶性不對稱,見於16個乳腺中6個(37.5%)。17個病灶中有4個(23.5%)經細針抽吸活檢確診。所有24個超聲引導的核心活檢均提供診斷。在四個樣本中發現棒狀桿屬菌(12.9%)。16名患者(55.2%)接受類固醇和抗生素聯合內科治療。18個病灶(58.1%)進行了引流。中位隨訪時間為21個月,當中13名患者(44.8%)復發。
結論
IGM是一種罕見良性慢性炎症性乳腺疾病,沒有特定的臨床或影像學特徵。應根據臨床表現通過活檢作出診斷。
INTRODUCTION
Idiopathic granulomatous mastitis (IGM), also
known as granulomatous lobular mastitis, was first
described by Kessler and Wolloch[1] in 1972. It is an
uncommon benign chronic inflammatory disease of
the breast. It is of unknown aetiology and primarily
affects young women. Potential precipitating factors
include pregnancy, lactation, oral contraceptive use,
and hyperprolactinaemia. There is no well-established
aetiology. It is characterised histologically by chronic
granulomatous inflammation in the breast lobules
without caseous necrosis. It should be differentiated
from other causes of granulomatous breast disease such
as plasma cell mastitis, granulomatosis with polyangiitis,
sarcoidosis, foreign body reaction, tuberculosis, and
fungal infections.[2]
Clinically, IGM typically presents as a breast mass
that may be associated with mastalgia, skin changes
such as thickening and sinus formation, or axillary
lymphadenopathy,[3] which cannot be distinguished from
breast cancer or infection.
In this retrospective study, we reviewed cases of IGM
in a tertiary referral hospital in Hong Kong. The aim
was to analyse the clinical, imaging, and pathological
features of IGM and discuss the available treatment
options.
METHODS
This retrospective study was approved by Hong Kong
West Cluster Research Ethics Committee and the
requirement to obtain informed consent was waived.
All consecutive patients who were diagnosed with IGM
between July 2012 and June 2019 were identified from
the hospital Radiological Information System.
Patient medical records were extracted from Electronic
Patient Records and Radiological Information System to
determine the clinical manifestations, pathological and
microbiological results, imaging findings, and treatment
plans.
Ultrasound examination of all involved breasts and
axillae was performed with a linear 5.5-18 MHz probe
(18L6 HD Transducer; Siemens, Erlangen, Germany).
Mammography was performed in patients with
suspicious ultrasound findings where appropriate. All
imaging studies and procedures were performed by
fellowship-trained breast radiologists. Tissue diagnosis
was obtained by fine needle aspiration biopsy (FNAB),
percutaneous ultrasound-guided core biopsy, or surgical
excision under ultrasound guidance.
RESULTS
Clinical Manifestations
Between July 2012 and June 2019, a total of 29 women (31 breasts) with a median age of 42 years (range, 25-58)
had breast imaging performed in our institution and were
subsequently diagnosed with IGM. Of the 29 women, 24
(82.8%) were of childbearing age. Five women (17.2%)
were postpartum. Nine (31%) had been breastfeeding
within the past year, with a duration ranging from
2 months to 2 years.
The reported duration of symptoms ranged from 3 to
56 days, with a median of 25 days. Common clinical
presentations included breast mass, swelling, mastalgia,
and erythematous skin changes, all of which can be seen
in breast abscess or cancer. Associated ulceration and
discharge were presented in two patients. Among the
29 patients, two were initially given an ultrasonographic
and/or mammographic diagnosis of breast cancer, while
22 were initially diagnosed and managed as breast
abscesses.
Nine (31%) patients were documented to have
hyperprolactinaemia. Two were due to pituitary
prolactinomas and four were due to antipsychotic drugs.
Imaging Findings
Among the 31 breasts included in the study, 31 received
ultrasound examinations and 16 of them received
mammography (Table).
Table. Ultrasound and mammographic findings of idiopathic
granulomatous mastitis
Among the 31 ultrasound examinations, 17 were
reported as irregular hypoechoic masses with or without
tubular extensions (Figure 1). The rest of the documented
features were one circumscribed hypoechoic mass
(Figure 2), 13 collections (Figure 3) and one parenchymal distortion with no discrete mass. Increased vascularity
was observed in nine of the cases. Associated ultrasound
findings included skin thickening (n = 11), axillary
lymphadenopathy (n = 4), sinus tract formation (n = 4)
(Figure 4) and nipple retraction (n = 2).
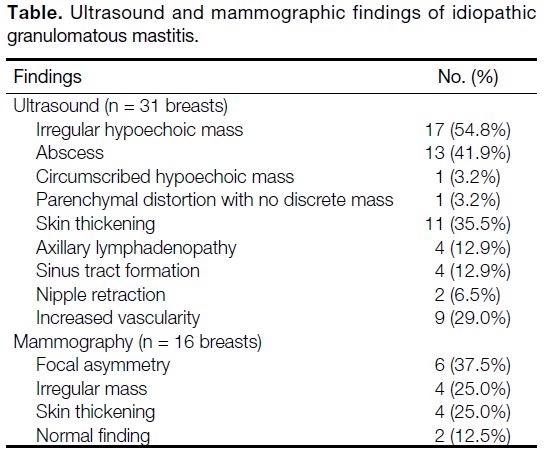
Figure 1. A 34-year-old woman who presented with right breast
mass. Ultrasound image of the right breast demonstrated a 4.4 cm
× 2.3 cm ill-defined irregular heterogeneous hypoechoic mass with
tubular extensions.
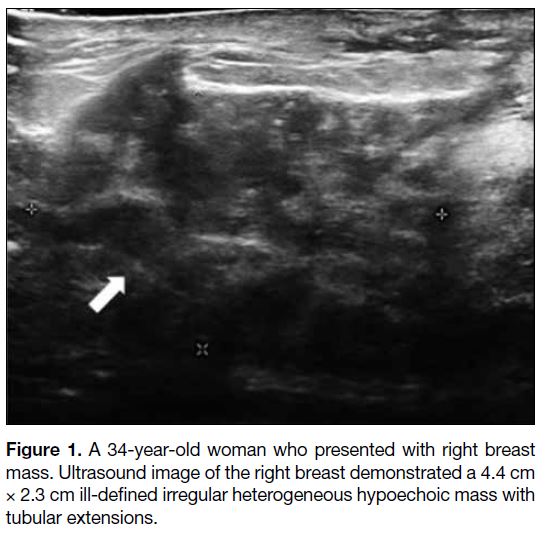
Figure 2. A 32-year-old woman who presented with a left breast
lump. Ultrasound image demonstrated a well-circumscribed
hypoechoic mass at 9 o’clock of the left breast, 3 cm from nipple.
Core biopsy confirmed idiopathic granulomatous mastitis.
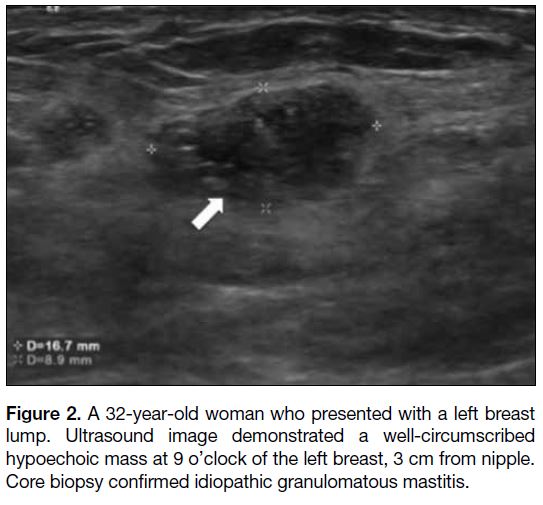
Figure 3. Ultrasound images showing irregular hypoechoic
collection in two patients with idiopathic granulomatous mastitis
mimicking breast abscess. (a) A 34-year-old woman presented with
erythema and mastalgia over the right breast. Ultrasound image
shows a collection at 12 o’clock of the right breast, 1 cm from the
nipple. Skin thickening was observed. 3 mL of blood stained fluid
was aspirated. The patient was initially treated as breast abscess.
(b) A 32-year-old woman presented with left mastalgia and skin
erythema. Ultrasound image shows a collection (arrow). Aspiration
yielded 2 mL of blood-stained fluid and patient was initially treated
as having a breast abscess.
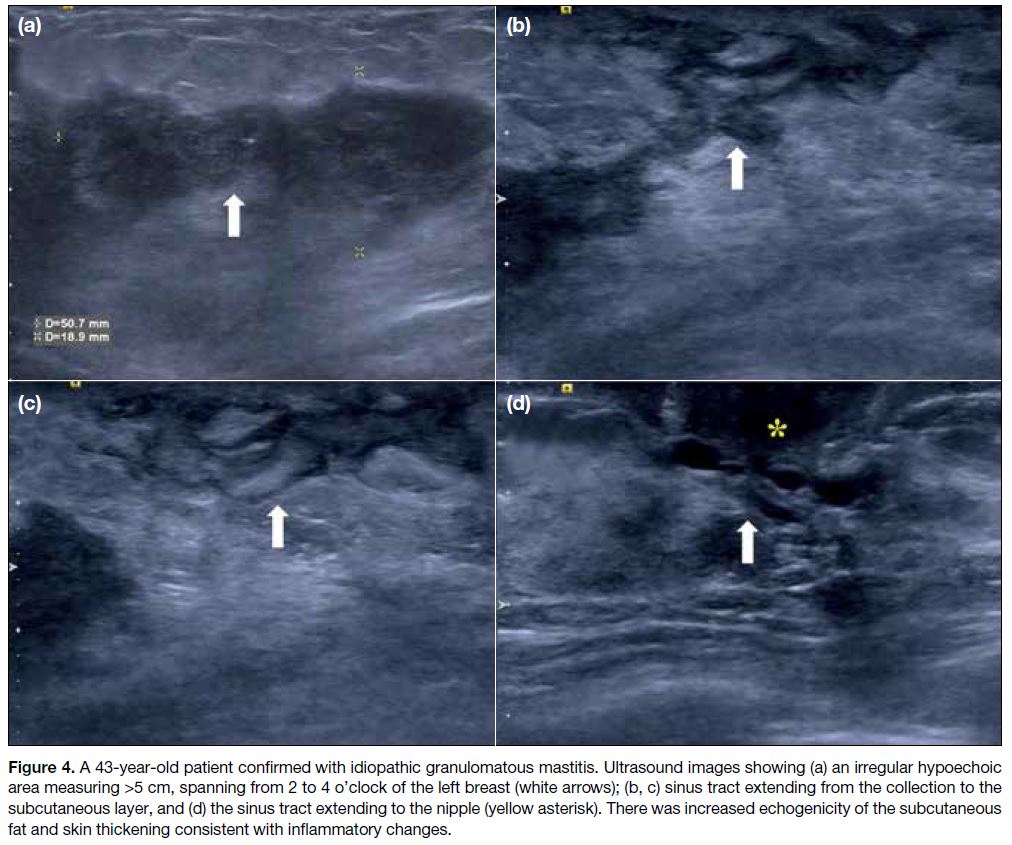
Figure 4. A 43-year-old patient confirmed with idiopathic granulomatous mastitis. Ultrasound images showing (a) an irregular hypoechoic
area measuring >5 cm, spanning from 2 to 4 o’clock of the left breast (white arrows); (b, c) sinus tract extending from the collection to the
subcutaneous layer, and (d) the sinus tract extending to the nipple (yellow asterisk). There was increased echogenicity of the subcutaneous
fat and skin thickening consistent with inflammatory changes.
Among the 16 mammogram examinations, the most
common finding was focal asymmetric density (n = 6).
Other findings included irregular masses (n = 4)
(Figure 5) and skin thickening (n = 4). Two mammograms
were negative.
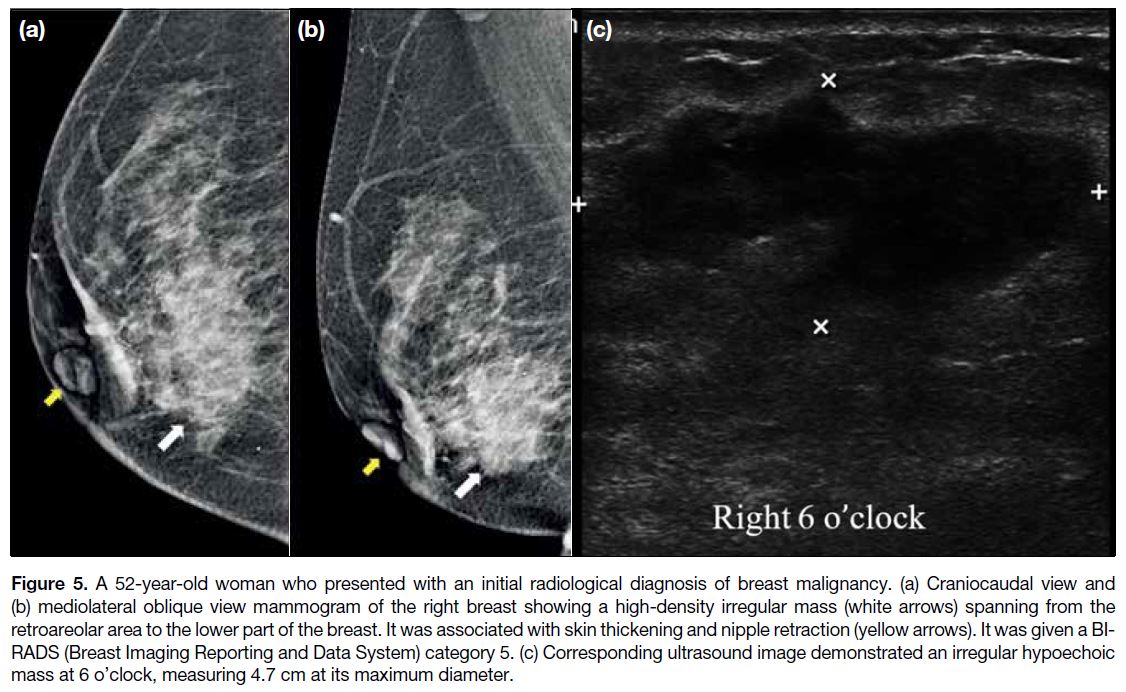
Figure 5. A 52-year-old woman who presented with an initial radiological diagnosis of breast malignancy. (a) Craniocaudal view and
(b) mediolateral oblique view mammogram of the right breast showing a high-density irregular mass (white arrows) spanning from the
retroareolar area to the lower part of the breast. It was associated with skin thickening and nipple retraction (yellow arrows). It was given a BI-RADS
(Breast Imaging Reporting and Data System) category 5. (c) Corresponding ultrasound image demonstrated an irregular hypoechoic
mass at 6 o’clock, measuring 4.7 cm at its maximum diameter.
Pathological Diagnosis and Evaluation
In total, 17 FNABs, 24 ultrasound-guided core biopsies,
and two surgical biopsies were performed, and were
diagnostic in 23.5%, 100% and 100%, respectively.
Microbiological testing was performed on all specimens
to exclude infectious causes, including tuberculosis and
fungal infection. Corynebacterium species were found in
four specimens.
Treatment and Follow-up
Sixteen patients were treated medically with a
combination of steroids and antibiotics, three were
treated with steroid monotherapy, and five did not receive any medical treatment. Drainage was performed in
18 lesions. One lesion was surgically excised.
Recurrence developed in 13 patients (44.8%) with a
median follow-up of 21 months.
DISCUSSION
Imaging features of IGM are nonspecific without
pathognomonic findings. The most frequently reported
ultrasound finding is an irregular hypoechoic mass
associated with multiple tubular extensions.[4] [5] [6] Fluid
collections or abscesses have also been described, with
a reported prevalence ranging from 6.6% to 54%.[7] Less
commonly described finding was parenchymal distortion
without a discrete mass.[2] [8] Associated findings such as
skin thickening, oedema, axillary adenopathy, and
sinus tract formation have also been observed.[2] [4] [9] In
our centre, ultrasound is the first assessment modality
for symptomatic patients aged <40 years. It is the
preferred modality for assessment of breast masses in
young Asian women, since mammography is considered
less sensitive than ultrasound in Asian populations,
where heterogeneous or extremely dense breast tissue
is common.[10] In our institution, ultrasound findings
for IGM were likewise nonspecific, with two patients
initially diagnosed with breast cancer and 22 patients
initially diagnosed and treated as breast abscess.
The commonly reported mammographic findings are
focal asymmetry and masses with irregularly shaped or
obscured margin.[7] Lee et al[11] reported other associated
findings including parenchymal distortion, skin
thickening, and axillary lymphadenopathy in 54.5% to
63.7% of patients. In our study, apart from the similarly
described features, two out of 16 of the lesions were
mammographically occult. This may be due to decreased
mammographic sensitivity in dense breasts, or poor
mammographic sensitivity in detection of IGM.
Due to the nonspecific radiological findings, biopsy
and histology are key to making the diagnosis of
IGM. Demonstration of non-caseous granulomatous
inflammation is required for definitive diagnosis of IGM.
Biopsy techniques include FNAB, core needle biopsy,
and surgical biopsy. Because of its ready availability
and low risk, FNAB is always performed to exclude
infection and malignancy. However, FNAB did not
provide sufficient tissue for diagnosis in these cases.
Core biopsy obtained sufficient specimens for diagnosis.
In our study, only 23.5% of the FNAB specimens were
diagnostic, while core biopsy yielded 100% diagnostic
quality.
IGM is a rare condition with no well-established
aetiology. Association with hyperprolactinaemia had
been postulated, in which prolactin-secreting pituitary
adenomas and antipsychotic medications were the most
common documented causes.[9] [12] [13] [14] [15] [16] Recently, Co et al[17]
proposed Corynebacterium kroppenstedtii as a risk
factor for IGM; it was associated with a higher rate of
disease recurrence. C kroppenstedtii is a slow-growing
opportunistic organism that rarely causes infection and
cannot be isolated using routine culture methods, that
often leads to underdiagnosis. Wong et al[18] postulated a
possible association between antipsychotic drug-induced
hyperprolactinaemia and C kroppenstedtii–related
mastitis. In our study, nine patients were documented
to have hyperprolactinaemia, in which C kroppenstedtii
was isolated in four of the specimens. However, prolactin levels are not investigated routinely in our
patients diagnosed with IGM and taking antipsychotic
medications; so hyperprolactinaemia could be
underdiagnosed. In view of possible association between
hyperprolactinaemia and C kroppenstedtii–related
mastitis, prolactin levels and C kroppenstedtii should be
investigated in patients diagnosed with IGM. Appropriate
treatment for hyperprolactinaemia and C kroppenstedtii
infection could be started as soon as possible to shorten
the disease course and decrease recurrence.
As IGM is a rare condition which lacks large cohort studies,
and definitive treatment strategies have yet to be established.
Conservative approaches such as antibiotics and drainage,
steroids and immunomodulatory drugs, as well as
surgical excision have been described in the literature.[19] [20] [21] [22]
Our study has some limitations because all clinical and
imaging findings were retrospectively reviewed. First,
some clinical data, such as breastfeeding history and use
of oral contraceptive, were missing; and investigations
including blood prolactin level and microbiological
testing were not performed in all patients. Therefore,
the role of these possible contributing factors in IGM
remains speculative. Second, mammography was
not performed in all of our patients. The decision of
performing mammography depended on patient age and
presentation, clinician preference and referral, ultrasound
findings, and operating radiologists’ preference. Future
multicentre studies with larger sample size are required
to clarify the relationship between blood prolactin levels
and IGM, and to compare the different imaging findings
in patients with IGM with or without C kroppenstedtii
infection.
CONCLUSION
In conclusion, IGM is a rare benign inflammatory
breast disease diagnosed by exclusion. It mimics breast
abscess or malignancy both clinically and radiologically.
There are no pathognomonic ultrasonographic or
mammographic findings. Therefore, histopathological
confirmation is important in disease diagnosis and management. Elevated prolactin levels and the presence
of C kroppenstedtii are possible risk factors for IGM and
should be investigated further.
REFERENCES
1. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically
simulating carcinoma. Am J Clin Pathol. 1972;58:642-6. Crossref
2. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G.
Granulomatous lobular mastitis: imaging, diagnosis, and treatment.
AJR Am J Roentgenol. 2009;193:574-81. Crossref
3. Akcan A, Akyildiz H, Deneme MA, Akgun H, Aritas Y.
Granulomatous lobular mastitis: a complex diagnostic and
therapeutic problem. World J Surg. 2006;30:1403-9. Crossref
4. Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, Alavi A,
Hemmati H, Esmaeili Delshad MS, et al. Granulomatous mastitis:
presentation, diagnosis, treatment and outcome in 206 patients from
the north of Iran. Breast. 2015;24:456-60. Crossref
5. Yildiz S, Aralasmak A, Kadioglu H, Toprak H, Yetis H, Gucin Z,
et al. Radiologic findings of idiopathic granulomatous mastitis.
Med Ultrasound. 2015;17:39-44. Crossref
6. Fazzio RT, Shah SS, Sandhu NP, Glazebrook KN. Idiopathic
granulomatous mastitis: imaging update and review. Insights
Imaging. 2016;7:531-9. Crossref
7. Pluguez-Turull CW, Nanyes JE, Quintero CJ, Alizai H, Mais DD,
Kist KA, et al. Idiopathic granulomatous mastitis: manifestations
at multimodality imaging and pitfalls. Radiographics. 2018;38:330-56. Crossref
8. Dursun M, Yilmaz S, Yahyayev A, Salmaslioglu A, Yavuz E,
Igci A, et al. Multimodality imaging features of idiopathic
granulomatous mastitis: outcome of 12 years of experience. Radiol Med. 2012;117:529-38. Crossref
9. Gautier N, Lalonde L, Tran-Thanh D, El Khoury M, David J,
Labelle M, et al. Chronic granulomatous mastitis: imaging,
pathology and management. Eur J Radiol. 2013;82:e165-75. Crossref
10. Handa P, Leibman AJ, Sun D, Abadi M, Goldberg A. Granulomatous
mastitis: changing clinical and imaging features with image guided
biopsy correlation. Eur Radiol. 2014;24:2404-11. Crossref
11. Lee JH, Oh KK, Kim EK, Kwack KS, Jung WH, Lee HK.
Radiologic and clinical features of idiopathic granulomatous
lobular mastitis mimicking advanced breast cancer. Yonsei Med
J. 2006;47:78-84. Crossref
12. Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous
mastitis: a 25-year experience. J Am Coll Surg. 2008;206:269-73. Crossref
13. Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A
clinicopathological review of 34 cases of inflammatory breast
disease showing an association between corynebacteria infection
and granulomatous mastitis. Pathology. 2003;35:109-19.
14. Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous
mastitis associated with risperidone-induced hyperprolactinemia.
Diagn Pathol. 2012;7:2. Crossref
15. Rowe PH. Granulomatous mastitis associated with a pituitary prolactinoma. Br J Clin Pract. 1984;38:32-4.
16. Erhan Y, Veral A, Kara E, Ozdemir N, Kapkac M, Ozdedeli E, et al.
A clinicopathologic study of a rare clinical entity mimicking breast
carcinoma: idiopathic granulomatous mastitis. Breast. 2000;9:52-6. Crossref
17. Co M, Cheng VC, Wei J, Wong SC, Chan SM, Shek T, et al.
Idiopathic granulomatous mastitis: a 10-year study from a
multicentre clinical database. Pathology. 2018;50:742-7. Crossref
18. Wong SC, Poon RW, Chen JH, Tse H, Lo JY, Ng TK, et al.
Corynebacterium kroppenstedtii is an emerging cause of mastitis
especially in patients with psychiatric illness on antipsychotic
medication. Open Forum Infect Dis. 2017;4:ofx096. Crossref
19. DeHertogh DA, Rossof AH, Harris AA, Economou SG.
Prednisone management of granulomatous mastitis. N Eng J Med.
1980;308:799-800. Crossref
20. Ocal K, Dag A, Turkmenoglu O, Kara T, Seyit H, Konca K.
Granulomatous mastitis: clinical, pathological features, and
management. Breast J. 2010;16:176-82. Crossref
21. Asoglu O, Ozmen V, Karanlik H, Tunaci M, Cabioglu N, Igci A,
et al. Feasibility of surgical management in patients with
granulomatous mastitis. Breast J. 2005;11:108-14. Crossref
22. Taghizadeh R, Shelley OP, Chew BK, Weiler-Mithoff EM.
Idiopathic granulomatous mastitis: surgery, treatment, and
reconstruction. Breast J. 2007;13:509-13. Crossref