Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Ventricular Noncompaction Cardiomyopathy
ORIGINAL ARTICLE CME
Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Ventricular Noncompaction Cardiomyopathy
S Altay
Department of Radiology, Izmir Ataturk Research and Training Hospital, Polat Caddesi, Turkey
Correspondence: Dr S Altay, Department of Radiology, Izmir Ataturk Research and Training Hospital, Polat Caddesi, Turke. Email: sedataltay@yahoo.com
Submitted: 6 Nov 2020; Accepted: 11 Dec 2020.
Contributors: The author designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript
for important intellectual content. The author had full access to the data, contributed to the study, approved the final version for publication, and
takes responsibility for its accuracy and integrity.
Conflicts of Interest: The author has disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: Izmir Katip Celebi University Non-Interventional Clinical Research Ethics Committee approved this retrospective study and
waived the need for informed consent because of the retrospective evaluation of anonymised medical data (Ref: 2020-743).
Abstract
Objective
A low left ventricular ejection fraction (LVEF) is the most important marker of a poor clinical prognosis
in many heart diseases. This study aimed to determine the cardiac magnetic resonance imaging (MRI) findings most
associated with reduced LVEF in left ventricular noncompaction cardiomyopathy (LVNC).
Methods
MRI scans of cases of LVNC with isolated left ventricular involvement were reviewed. The number of
segments involved by LVNC, LVEF, wall thickness of LVNC-affected segments, cardiac output, and cardiac index
were determined. An LVEF value of ≤30% was accepted as a poor prognosis indicator. Low LVEF and morphology
were compared by the Pearson correlation method.
Results
In total, 31 cases of LVNC were included. The highest correlation was found between EF and the number of
segments affected (r = -0.6). LVEF was inversely correlated with the number of segments affected. Other factors that
were significantly associated with low LVEF were the presence of fibrosis, low cardiac output, and low cardiac index.
Conclusion
In conclusion, the number of non-compact segments is the most important morpho-pathological factor in clinical follow-up and treatment planning in LVNC cases due to its effect on EF change.
Key Words: Cardiomyopathies; Heart; Magnetic resonance imaging; Myocardium; Prognosis
中文摘要
心臟磁共振在心室緻密化不全心肌病的診斷和預後的作用
S Altay
目的
低左心室射血分數(LVEF)是許多心臟病臨床預後不良的最重要標誌。本研究旨在明確與左心室緻密化不全心肌病(LVNC)中LVEF降低最相關的心臟磁共振成像(MRI)表現。
方法
回顧分析孤立左心室受累LVNC病例的MRI掃描。測定LVNC涉及的節段數、LVEF、LVNC受累節段的壁厚、心輸出量和心臟指數。LVEF值30%或以下被認為是預後不良的指標。採用 Pearson相關分析比較低 LVEF值和形態學改變。
結果
共納入31例 LVNC。在射血分數和受影響的段數之間看到最高相關性 (r = -0.6)。LVEF與受影響的節段數呈負相關。與低LVEF顯著相關的其他因素包括存在纖維化、低心輸出量和低心指數。
結論
非緻密節段的數量是LVNC病例臨床隨訪和治療計劃中最重要的形態病理因素,因為它對射血分數變化有影響。
INTRODUCTION
Left ventricular noncompaction cardiomyopathy
(LVNC) is a rare disease that occurs as a result of failure
of the process of trabeculation in the left ventricular (LV)
wall to proceed after 5 to 8 weeks of foetal development.[1]
It is characterised by the presence of a subendocardial
non-compacted layer. A non-compacted layer >66%
of the entire wall thickness is diagnostic of LVNC.[1] [2]
The apex and inferolateral wall are the segments most
affected in the left and, sometimes, the right ventricle.[1]
Resulting heart failure, thromboembolism, arrhythmia,
or sudden death can occur.[2] Microvascular dysfunction
and ischaemia play a role in the pathogenesis of
arrhythmia and/or LV dysfunction.[3] The European
Society of Cardiology includes LVNC in the group of
unclassified cardiomyopathy as it is unclear whether it
is discrete cardiomyopathy or merely a morphological
feature shared by many different phenotypic
cardiomyopathies, but the American Society of
Cardiology considers LVNC primary cardiomyopathy.[3]
There are different opinions as to the aetiology. Genetic
and sporadic causes are found in the literature.[4] [5]
Significant genetic heterogeneities have been identified
in LVNC, but the familial major genetic cause has not
yet been identified.[5]
Data on the pathological findings affecting the clinical
course in LVNC cases are insufficient in the literature.[6]
In many studies, LVNC cases have been evaluated by
echocardiography, but morpho-pathological causes
affecting the prognosis of the disease have not been
clearly defined.[7] Morphological and functional data
may be obtained with cardiac magnetic resonance
(CMR) examinations in LVNC cases. We examined the
morphological and functional abnormalities of LVNC
with CMR.
A lower left ventricular ejection fraction (LVEF) than
normal values determine the clinical severity of LVNC
heart involvement. Correlations between CMR findings and LVEF change in LVNC cases were evaluated.
This study aimed to find out the effects of morpho-pathological
changes on prognosis in LVNC cases.
METHODS
Patients
A total of 63 cases diagnosed with LVNC in CMR
examination between 2014 and 2019 in our hospital’s
image archiving system were retrospectively re-evaluated.
For the diagnosis of LVNC, the Petersen
diagnostic criteria were used in cases with uncompressed
tissue thickness ≥66% of the total wall thickness.[8] [9]
Measurements were made on 2- and 4-chamber images
in the end-diastolic phase. Three cases with isolated
right ventricular involvement, 10 cases with coronary
artery disease, seven cases with right and left ventricular
involvement, five cases with dilated cardiomyopathy,
and seven cases with a history of cardiac surgery were
excluded from the study.
The remaining 31 cases with isolated LV involvement
and no known disease were included in the study.
Cases were included in the examination regardless of
clinical symptoms. Ejection fraction (EF), cardiac output
(CO), cardiac index (CI) values, and wall involvement
rate (%) as were calculated. The number of segments
affected by LVNC was calculated with the American
Heart Association’s 17-segment model. The data of the
patients were evaluated statistically independent of age.
Cardiac Magnetic Resonance Proto
CMR studies were performed on a 1.5 Tesla scanner
(Aera®; Siemens Healthineers, Erlangen, Germany).
Patients were scanned with 16-channel surface phased
array body coils. Cine images were acquired in the
2-chamber and 4-chamber views for the heart. Balanced
steady-state free precession cine imaging (time of
repetition/time of echo, 2.7-3.1/1.4-1.5; flip angle, 65°;
temporal resolution, 25-39 ms; in-plane resolution,
1.9 × 1.9 to 2.6 × 2.7 mm; mean (± standard deviation) value, 2.2 ± 0.2 × 2.2 ± 0.2 mm; breath-hold duration,
10 to 12 heartbeats per section acquired. The section
thickness was 8 mm with a gap of 2 mm) was performed
in long-axis 2- and 4-chamber views for biplanar
assessment of LV end-diastolic volume, LV mass,
and LVEF. Contours were drawn automatically, and if
needed, manually corrected. Biplanar anatomical and
functional parameters were calculated automatically by
post-processing on a workstation (Syngo; Siemens)
We administered 0.2 mmol/kg intravenous gadopentetate
dimeglumine (Magnevist; Schering, Berlin, Germany)
via the antecubital vein for late gadolinium enhancement
imaging. A flow rate of 2 mL/sec was used. A minimum
of 10 minutes after contrast administration, and
inversion-recovery steady-state free precession inversion
time (TI) short-axis scouting sequence was acquired at
the mid-ventricular level to determine optimal TI. TI was
calculated for optimal suppression of myocardial signal.
Short- and long-axis late gadolinium enhancement
images including LV myocardium were acquired using
the FLASH-PSIR sequence.
Imaging Analysis
CMR examinations were evaluated by a radiologist who
has a cardiac imaging certificate with extensive CMR
experience (>9 years). EF, stroke volume, CI, CO, heart muscle weight, and LV peak ejection values were
calculated automatically over functional sequences in
the workstation. In 2- and 4-chamber images the end-diastolic
wall thicknesses were measured manually as a
full layer and a non-compacted layer. The presence of
myocardial fibrosis was analysed as present or absent
regardless of segment size.
Statistical Analysis
Consequently, the independent variable with a p value
< 0.05 will remain in the final model. The existence of
a dependent variable (EF) was investigated. Pearson
correlation evaluation was performed to determine the
linear relationship between the two continuous variables
with EF and LVNC involved wall ratio, CO, and CI
change. If the found r value was -1, it was interpreted as
a fully negative linear relationship, while +1 was a fully
positive linear relationship. An r = 0 meant no linear
relationship between the two variables. The closer the
absolute value of the correlation coefficient was to the
value of 1, the stronger was the linear association.
RESULTS
The age range of the 31 cases was between 15 and
56 years (mean = 45 ± 20). A total of 25 patients had
a known diagnosis of LVNC and six patients presented
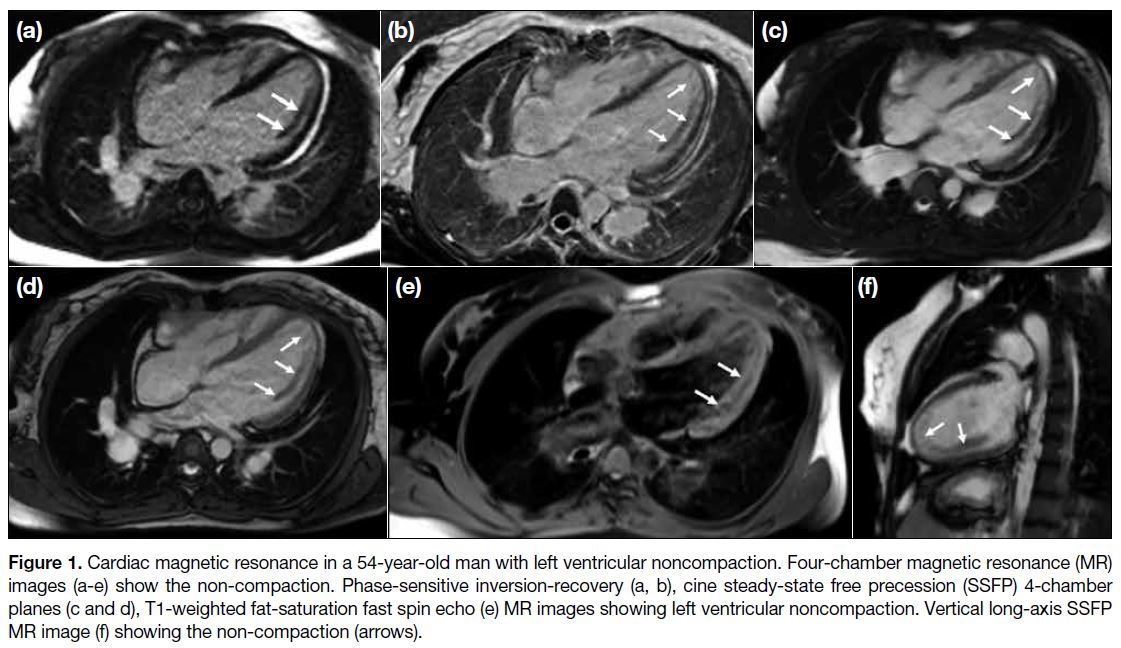
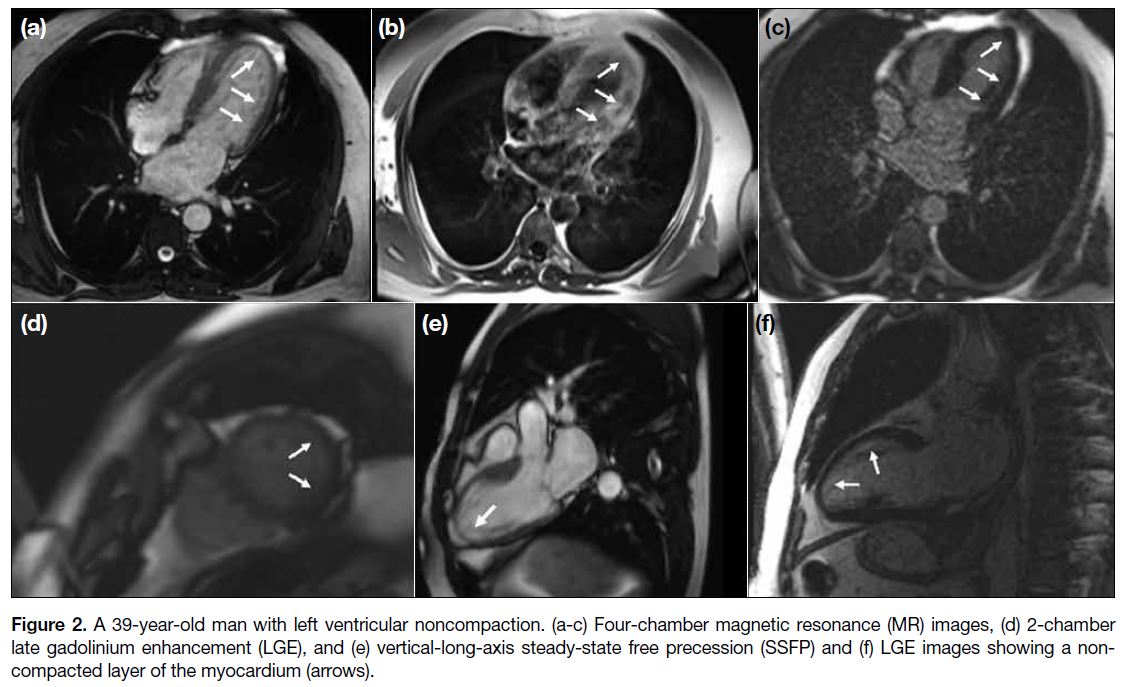
with increased trabeculation (Figures 1 and 2).
Figure 1. Cardiac magnetic resonance in a 54-year-old man with left ventricular noncompaction. Four-chamber magnetic resonance (MR)
images (a-e) show the non-compaction. Phase-sensitive inversion-recovery (a, b), cine steady-state free precession (SSFP) 4-chamber
planes (c and d), T1-weighted fat-saturation fast spin echo (e) MR images showing left ventricular noncompaction. Vertical long-axis SSFP
MR image (f) showing the non-compaction (arrows).
Figure 2. A 39-year-old man with left ventricular noncompaction. (a-c) Four-chamber magnetic resonance (MR) images, (d) 2-chamber
late gadolinium enhancement (LGE), and (e) vertical-long-axis steady-state free precession (SSFP) and (f) LGE images showing a noncompacted
layer of the myocardium (arrows).
Cardiac Magnetic Resonance Findings
The mean LVEF was 45.3% ± 35%, the number of
LVNC affected segments was 10 (range, 3-17), CO
was 5.7, and CI was 2.9. The mean stroke volume was
64.2 mL and heart muscle weight was 132.9 ± 35.23 g.
Segment involvement was the highest in segment 17
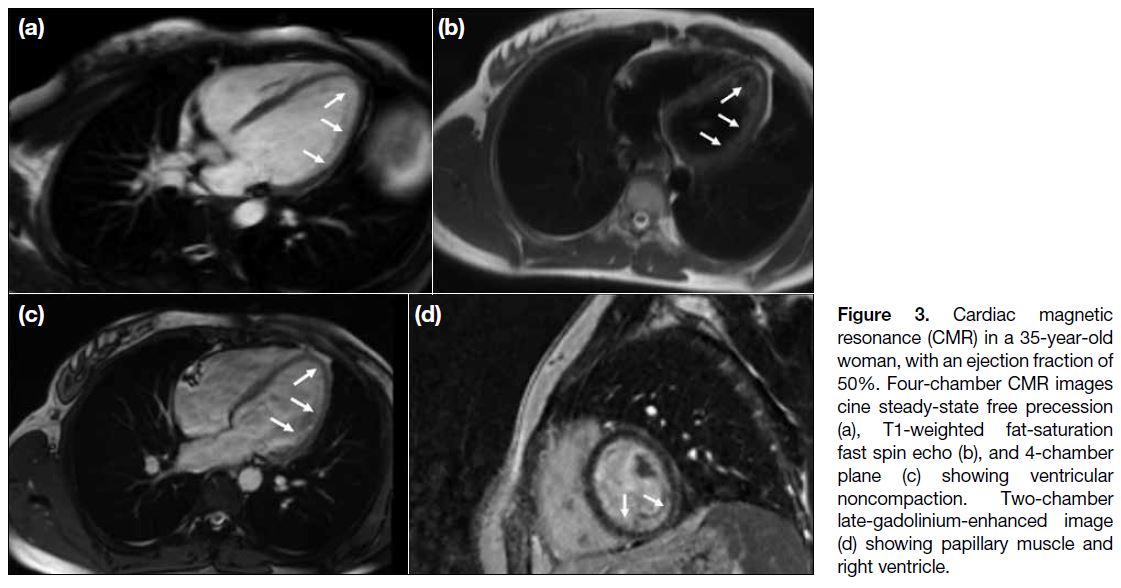
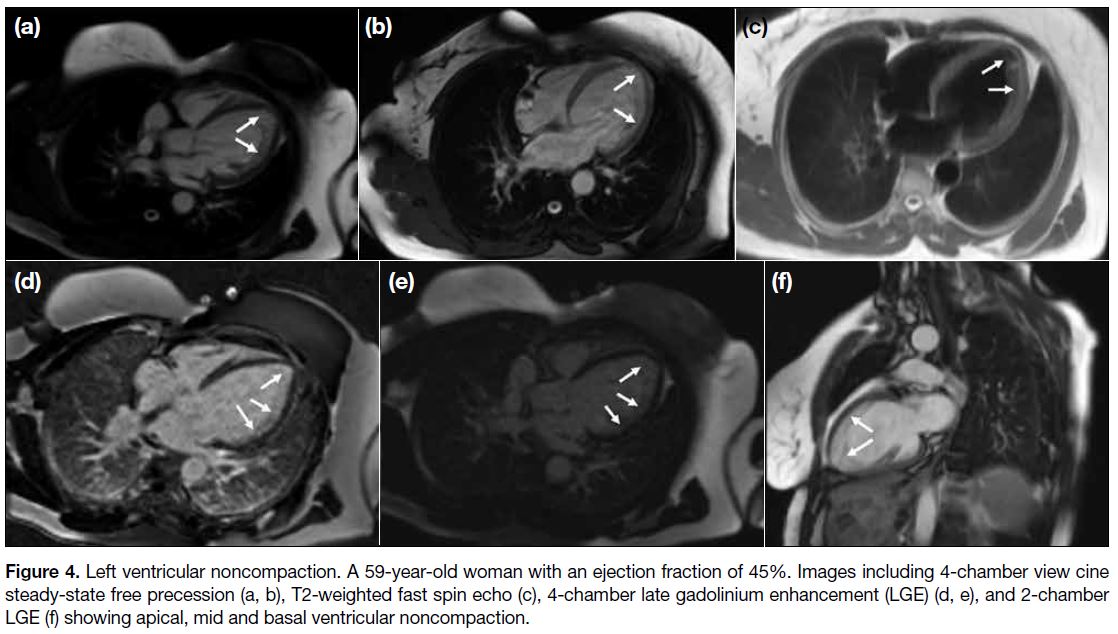
with the apex involved in 29 cases (Figures 3 and 4).
The involvement of apical segments 13 to 16, medial
segments 7 to 12, and basal segments 1 to 6 are shown
in Figures 5 and 6. Fibrosis in myocardial tissue was
detected in five cases. Additional LV findings in CMR
were a sigmoid septum in two cases, atrial septal defect
in one case, the false tendon anatomic variant in two
cases, LV lateral wall systolic hypokinesia in five cases,
first-degree mitral valve insufficiency in 10 cases, and
first-degree mitral valve stenosis in nine cases. Cor
triatriatum was observed in the right ventricle in one
case.
Figure 3. Cardiac magnetic
resonance (CMR) in a 35-year-old woman, with an ejection fraction of 50%. Four-chamber CMR images cine steady-state free precession (a), T1-weighted fat-saturation fast spin echo (b), and 4-chamber plane (c) showing ventricular noncompaction. Two-chamber late-gadolinium-enhanced image (d) showing papillary muscle and right ventricle.
Figure 4. Left ventricular noncompaction. A 59-year-old woman with an ejection fraction of 45%. Images including 4-chamber view cine
steady-state free precession (a, b), T2-weighted fast spin echo (c), 4-chamber late gadolinium enhancement (LGE) (d, e), and 2-chamber
LGE (f) showing apical, mid and basal ventricular noncompaction.
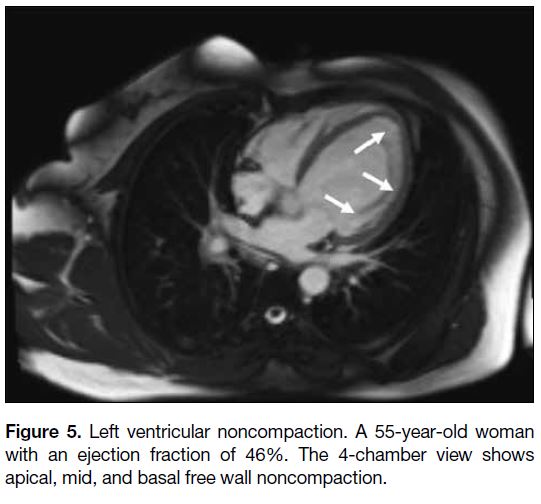
Figure 5. Left ventricular noncompaction. A 55-year-old woman
with an ejection fraction of 46%. The 4-chamber view shows
apical, mid, and basal free wall noncompaction.
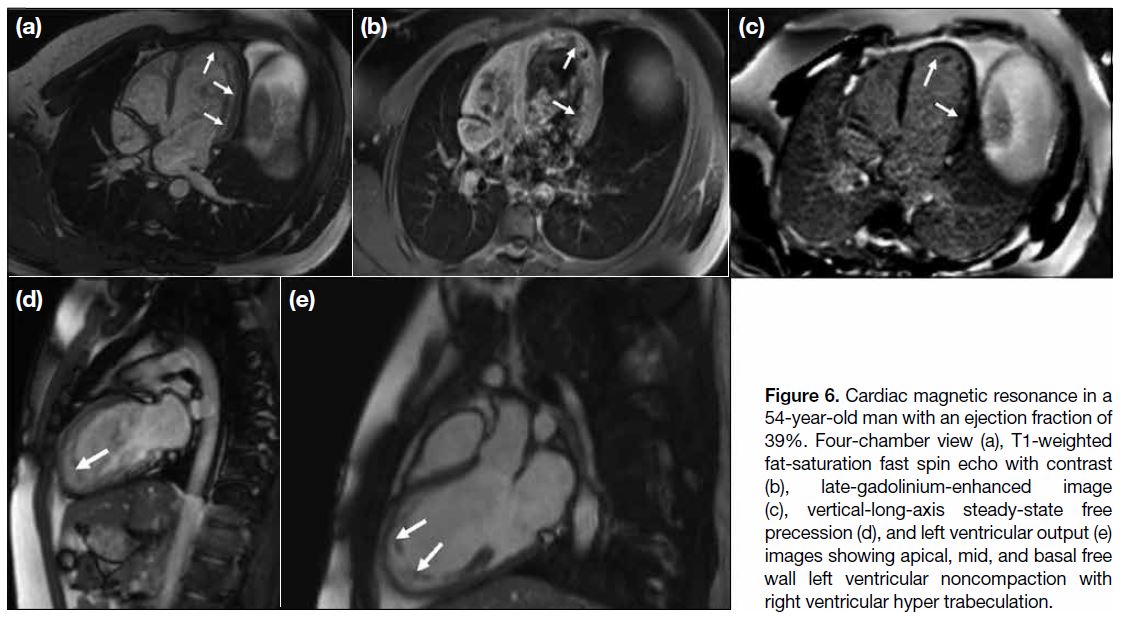
Figure 6. Cardiac magnetic resonance in a
54-year-old man with an ejection fraction of
39%. Four-chamber view (a), T1-weighted
fat-saturation fast spin echo with contrast
(b), late-gadolinium-enhanced image
(c), vertical-long-axis steady-state free
precession (d), and left ventricular output (e)
images showing apical, mid, and basal free
wall left ventricular noncompaction with
right ventricular hyper trabeculation.
Correlation between Ejection Fraction and
Other Cardiac Magnetic Resonance Findings
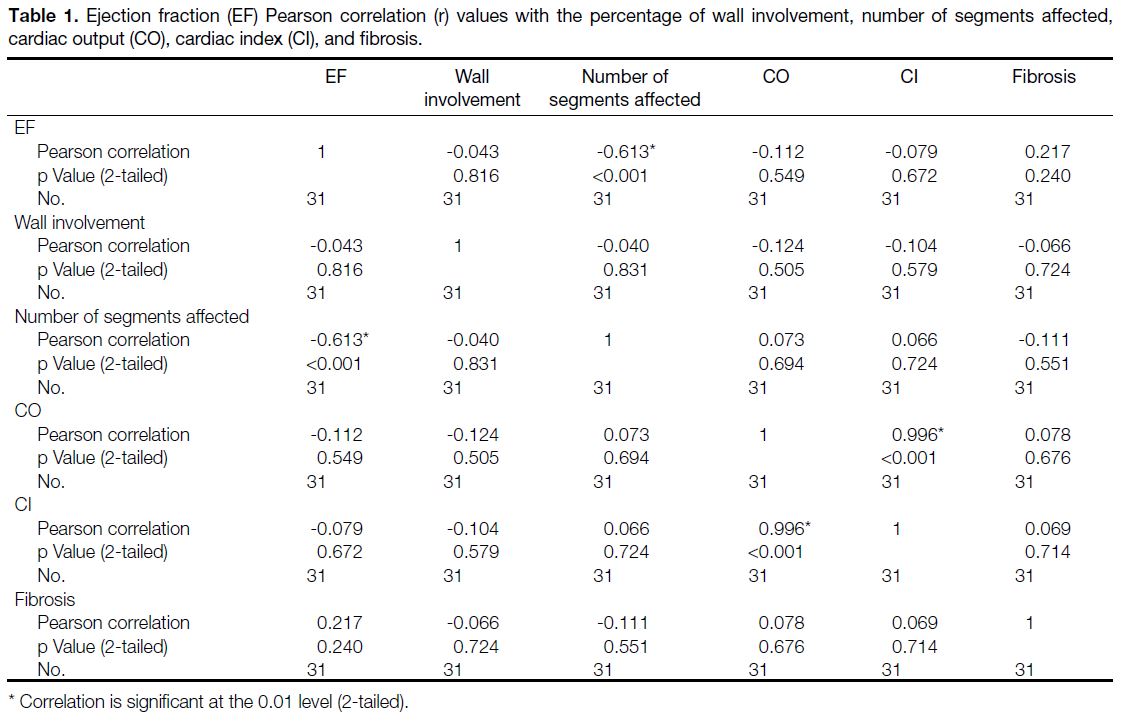
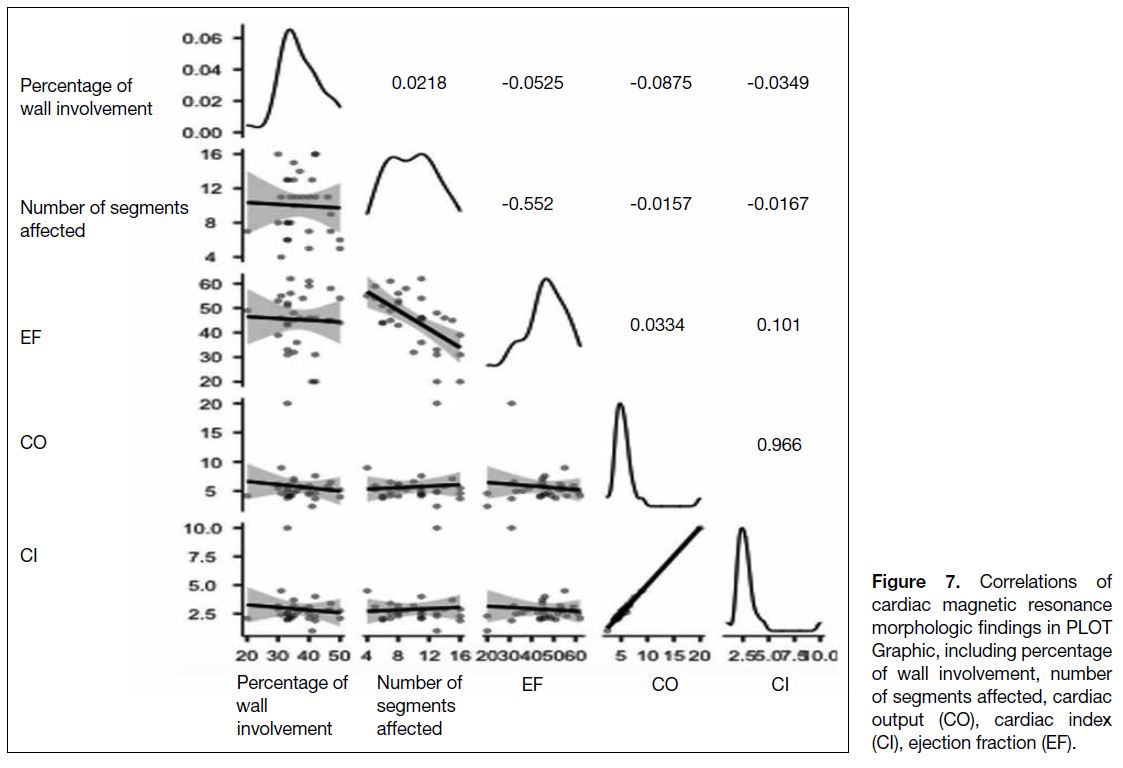
Correlation coefficients between EF and the following
findings are as follows: the number of affected segments
(r = -0.613), fibrosis (r = 0.217), CO (r = -0.112), CI (r = -0.079), and the percentage of non-compaction
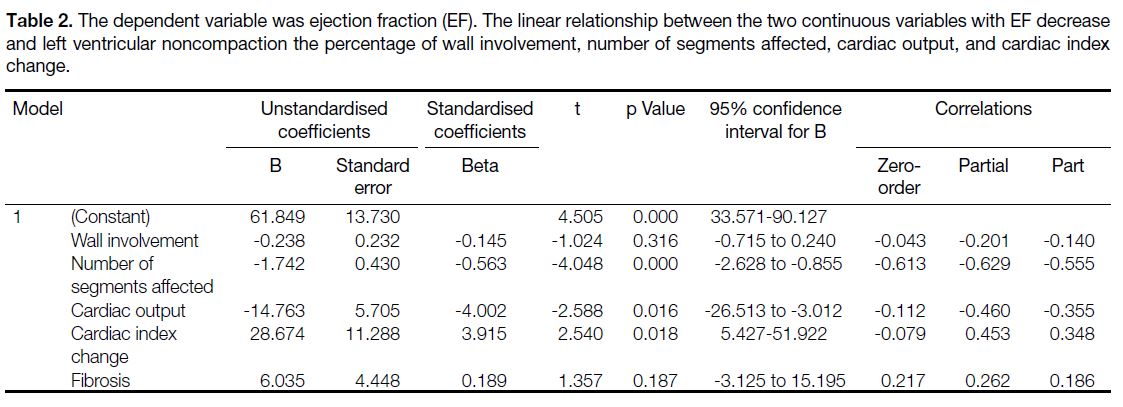
in the wall (r = -0.043) (Table 1 and Figure 7). The
most consistent factor affecting the EF was the number
of segments affected by LVNC (Table 2). There is no
multiple dependency variable in our variables. In the
evaluation made in terms of the statistical value of the
EF and Pearson correlation, the statistical value in the
number of segments held was found as p < 0.001, in the
presence of fibrosis as p = 0.240, with CO as p = 0.549,
with CI as p = 0.672 and the percentage of noncompaction
in the wall was found to be as p = 0.816.
Pearson correlation coefficient (r) value explanations
are made as there is no correlation if r < 0.2, a weak
correlation from 0.2 to 0.4, moderate correlation from 0.4
to 0.6, a high correlation from 0.6 to 0.8, and a very high
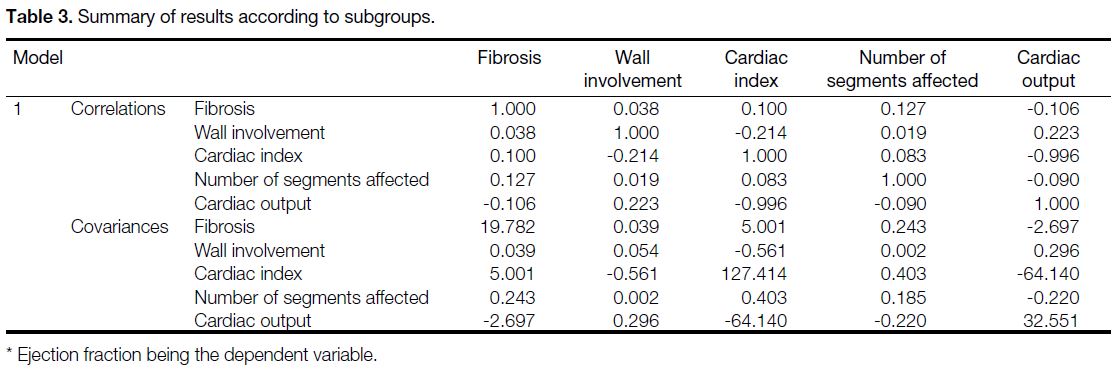
correlation from 0.8 to 1 (Tables 1 and 3). Statistically,
the finding affecting clinical severity in patients with
LVNC and highly correlated with prognosis was the
number of segments affected by the disease.
Table 1. Ejection fraction (EF) Pearson correlation (r) values with the percentage of wall involvement, number of segments affected,
cardiac output (CO), cardiac index (CI), and fibrosis.
Figure 7. Correlations of
cardiac magnetic resonance
morphologic findings in PLOT
Graphic, including percentage
of wall involvement, number
of segments affected, cardiac
output (CO), cardiac index
(CI), ejection fraction (EF).
Table 2. The dependent variable was ejection fraction (EF). The linear relationship between the two continuous variables with EF decrease
and left ventricular noncompaction the percentage of wall involvement, number of segments affected, cardiac output, and cardiac index
change.
Table 3. Summary of results according to subgroups.
DISCUSSION
This study aimed to define the morphological pathology
most closely linked to the prognosis in LVNC.
Knowledge regarding diagnosis, morbidity, and prognosis in this disease is limited.[8] CMR is recognised
as the gold standard for measuring the volume, mass,
and ejection fraction of both ventricles.[10] EF value is an important indicator of prognosis in coronary pathologies.
A low EF is a predictor of poor prognosis, except in
cases with preserved ejection fraction and heart failure.[7]
In this study, the morphological finding showing the
highest correlation with EF change was investigated. In
LVNC cases, the highest correlation was found between
low EF and the number of segments involved. In the
study, a high inverse correlation was observed between
the number of affected segments in LVNC and EF. In
conclusion, the number of affected segments in LVNC cases is the most influential factor in clinical follow-up
and treatment planning due to EF effects.
Low LVEF in LVNC cases is an important indicator
of a negative prognosis. Dodd et al[4] evaluated the
relationship between prognosis in myocardial fibrosis
and LVNC.[3] Negri et al[1] evaluated multiple studies
reporting associations between LVNC and ventricular
tachycardia. The prognosis was evaluated in LVNC with
a single variable in the studies the literature; this study
evaluated many morphological and functional factors
and consequently found the increase in the number of
affected segments corresponds most closely to low EF
in LVNC.
The 2016 European Society of Cardiology guidelines
for the diagnosis and treatment of heart failure defined
heart failure based on LVEF. The decrease in EF may
manifest itself as heart failure. Heart failure can develop
in LVNC cases.[10] Morpho-pathological factors affecting
this process have not been defined as multivariate in
the literature before. This study showed the highest
correlation with the decreasing LVEF in the LVNC
cases the number of involved segments. The number
of affected segments must be the most important factor
for clinical follow-up and treatment planning in LVNC
cases after these results.
Limitations of the Study
Our study design had a few limitations. First, there were
no histologically confirmed cases of LVNC. Another
limitation of our study was that it was performed with
a small number of patients. Furthermore, because this
review was retrospective, the clinical information was
incomplete. LVNC is rare cardiomyopathy that is not fully
classified.[11] Involvement is not homogeneous throughout
the heart. The patient group was heterogeneous in terms
of the diagnostic methodology. Long-term follow-up
is required to determine the prognosis in LVNC cases.
Long-term follow-up was not possible in the study.
No age grouping was made since our cases were few.
Another limitation was that the study was a single-centre
study and all CMR examinations were interpreted by
a single radiologist. Current criteria for the diagnosis
of LVNC lead to highly variable disease prevalence in
patients referred for CMR. Petersen criteria were used in
this study.[1] [9] [12] [13]
CONCLUSION
A high correlation was found between the number of
segments involved and a poor prognosis in LVNC cases.
The number of preserved segments correlates positively
with LVEF and is the most influential anatomical
factor in the clinical severity of the cases in the clinical
prognosis and treatment planning of LVNC cases. In
the CMR examination by radiologists, reporting the
number of preserved segments in LVNC patients will be
a guide to determining the appropriate treatment choice.
Prospective studies with larger patient series are needed
to determine the factors that play a role in the prognosis
of this disease.
REFERENCES
1. Negri F, De Luca A, Fabris E, Korcova R, Cernetti C, Grigoratos C,
et al. Left ventricular noncompaction, morphological, and clinical
features for an integrated diagnosis. Heart Fail Rev. 2019;24:315-23. Crossref
2. Peters F, Khandheria BK, dos Santos C, Matioda H, Maharaj N, Libhaber E, et al. Isolated left ventricular noncompaction in sub-Saharan Africa: a clinical and echocardiographic perspective. Circ Cardiovasc Imaging. 2012;5:187-93. Crossref
3. Mihailovici AR, Padureanu V, Albu CV, Dinescu VC, Pirlog MC,
Dinescu SN, et al. Myocardial noncompactation. Rev Chim.
2018;69:2209-12. Crossref
4. Dodd JD, Holmvang G, Hoffmann U, Ferencik M, Abbara S,
Brady TJ, et al. Quantification of left ventricular noncompaction
and trabecular delayed hyperenhancement with cardiac MRI:
correlation with clinical severity. AJR Am J Roentgenol.
2007;189:974-80. Crossref
5. Hussein A, Karimianpour A, Collier P, Krasuski R. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol. 2015;66:578-85. Crossref
6. Finsterer J, Stöllberger C, Towbin JA. Left ventricular
noncompaction cardiomyopathy: cardiac, neuromuscular, and
genetic factors. Nat Rev Cardiol. 2017;14:224-37. Crossref
7. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA.
Jenni R. Long-term follow-up of 34 adults with isolated left
ventricular noncompaction: a distinct cardiomyopathy with poor
prognosis. J. Am Coll Cardiol. 2000;36:493-500. Crossref
8. Jefferies JL, Chang AC, Rossano JW, Shaddy RE, Towbin JA,
editors. Heart Failure in the Child and Young Adult from Bench
to Bedside Memphis: Elsevier; 2018. p 269-90.
9. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am
Coll Cardiol. 2005;46:101-5. Crossref
10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG,
Coats AJ, et al. 2016 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure: the task force for the
diagnosis and treatment of acute and chronic heart failure of the
European society of cardiology (ESC). Developed with the special
contribution of the heart failure association (HFA) of the ESC. Eur
Heart J. 2016;37:2129-200. Crossref
11. Dreisbach JG, Mathur S, Houbois CP, Oechslin E, Ross H,
Hanneman K, et al. Cardiovascular magnetic resonance-based
diagnosis of left ventricular non-compaction cardiomyopathy:
impact of cine bSSFP strain analysis. J Cardiovasc Magn Reson.
2020;22:9. Crossref
12. Captur G, Muthurangu V, Cook C, Flett AS, Wilson R, Barinson A,
et al. Quantification of left ventricular trabeculae using fractal
analysis. J Cardiovasc Magn Reson. 2013;15:36. Crossref
13. Ivanov A, Dabiesingh DS, Bhumireddy GP, Mohamed A,
Asfour A, Briggs WM, et al. Prevalence and prognostic significance
of left ventricular noncompaction in patients referred for
cardiac magnetic resonance imaging. Circ Cardiovasc Imaging.
2017;10:e006174. Crossref