Translumbar Tunnelled Placement of a Haemodialysis Catheter in a Patient with Transposition of the Inferior Vena Cava: A Case Report
CASE REPORT
Translumbar Tunnelled Placement of a Haemodialysis Catheter in a Patient with Transposition of the Inferior Vena Cava:
A Case Report
T Jonszta, D Czerny, V Prochazka, V Chovanec, A Krajina
Radiology Department, Ostrava University Hospital, Ostrava, Czech Republic
Correspondence: Dr T Jonszta, Radiology Department, Ostrava University Hospital, Ostrava, Czech Republic. Email:
Submitted: 1 May 2020; Accepted: 25 Aug 2020.
Contributors: TJ designed the study. TJ and DC acquired data. TJ analysed the data. TJ, VP and VC drafted the manuscript. VP, VC and AK
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This case report was approved by the local ethics committee of the University Hospital Ostrava in April 2020. Informed consent was obtained from the patient.
INTRODUCTION
Inferior vena cava (IVC) transposition is a well-known
anatomic variant[1] with a reported prevalence of 0.2% to
0.5%.[2] Due to the complexity of IVC embryogenesis,
many anatomical forms and variations are encountered.
Anomalies of the IVC can be misdiagnosed and
overlooked but are usually visualised by cross-sectional
non-invasive imaging methods including computed
tomography (CT) and magnetic resonance imaging.[2] [3] In
most patients these variations are asymptomatic, but they
can be a potential cause of complications during surgical
or interventional radiological procedures.
CASE REPORT
A 59-year-old man with femoral haemodialysis catheter
failure due to iliac vein thrombosis was admitted for
placement of a translumbar tunnelled haemodialysis
catheter (TLC). His medical history included myocardial
infarction, hypertension, hyperlipidaemia, diabetes,
chronic kidney disease, and renal failure consequent to
diabetic nephropathy. He had been receiving dialysis
for 8 years, during which time he had required repeated catheter exchanges and been treated for repeated catheter-related
sepsis, and bilateral brachiocephalic and superior
vena cava occlusions. Bilateral femoral veins had been
previously catheterised with thrombotic complications
and catheter malfunction.
Informed consent was obtained and coagulation tests
were performed prior to the procedure. Catheter insertion
was performed in two steps. Under local anaesthesia
and mild conscious sedation with the patient in a
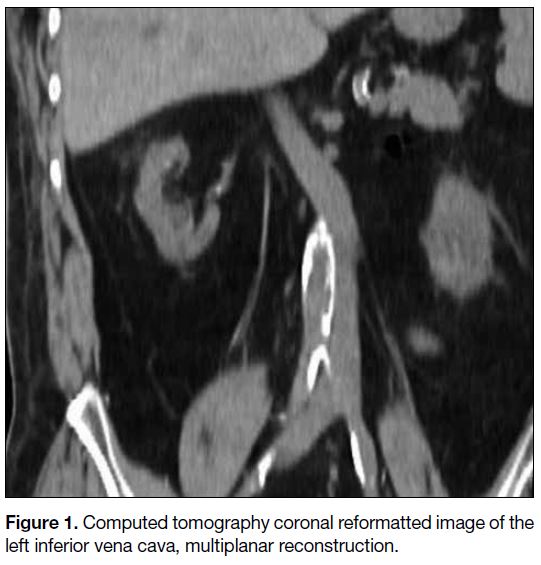
prone position, the access route was planned under CT
guidance. The left IVC was visualised so the puncture
needle track was switched from the right to the left side
(Figure 1). Navigation was performed by repeated CT
spiral scans with gradual advancement of the needle
from above the left iliac crest toward the infrarenal
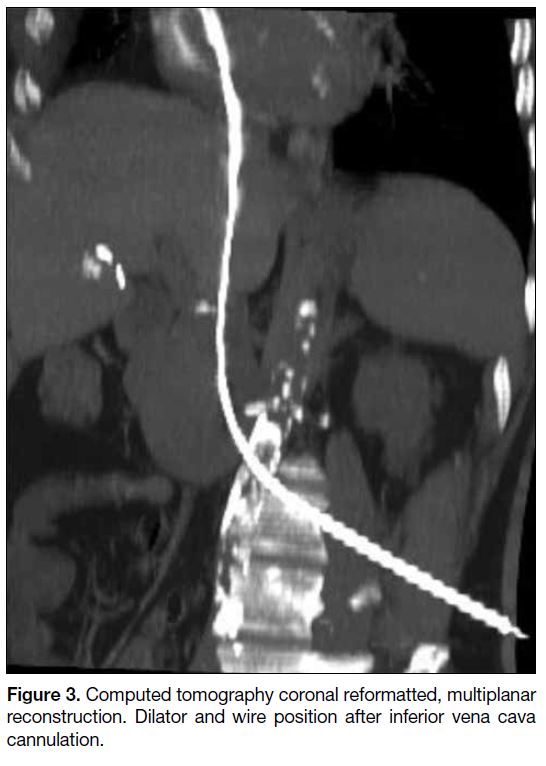
segment of the IVC (Figure 2). After IVC puncture, a
6F 33-cm dilator was inserted over the wire (Figure 3).
In the second step, the patient was transferred to the
angiography suite. Under fluoroscopic guidance and in
the right lateral decubitus position, subcutaneous tunnel
length and catheter trajectory were planned. A dedicated double lumen catheter for a translumbar approach (Split-
Cath® III; MedComp, Harleysville [PA], United States)
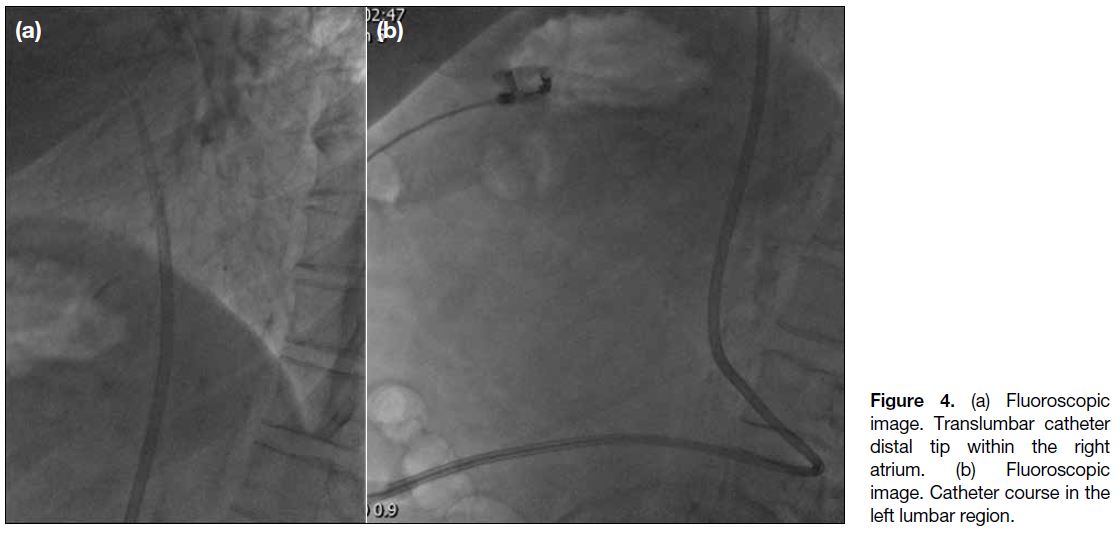
was tunnelled and inserted. Correct positioning of the
catheter tip in the right atrium and potential complications
were evaluated again on site with fluoroscopy (Figure 4).
After aspiration and flushing, 4% citrate lock Intralock®
Fresenius was administered. Antibiotic prophylaxis was
administered for 5 days. The procedure was uneventful
and haemodialysis was performed to test the function of
the catheter prior to discharge of the patient.
Figure 1. Computed tomography coronal reformatted image of the left inferior vena cava, multiplanar reconstruction.
Figure 2. (a) Computed tomography transverse image of the left
inferior vena cava (IVC). Patient in prone position. (b) Computed
tomography–navigated left IVC puncture. Patient in prone position.
Figure 3. Computed tomography coronal reformatted, multiplanar reconstruction. Dilator and wire position after inferior vena cava cannulation.
Figure 4. (a) Fluoroscopic
image. Translumbar catheter distal tip within the right atrium. (b) Fluoroscopic image. Catheter course in the left lumbar region.
DISCUSSION
Venous anomalies and variations of the IVC are
observed quite frequently but there is no consensus on
their classification. The most frequently encountered
and published anomalies include the retroaortic left
renal vein, left IVC, double IVC, circumaortic left
renal vein, interruption of the IVC with azygos and
hemiazygos continuation, absence of the infrarenal IVC
and circumcaval ureter.[1] [3]
The left-sided IVC results from regression of the
right supracardinal vein with persistence of the left
supracardinal vein. The IVC is then created by the
iliac veins junction and continues to the left renal vein
that crosses anterior to the aorta in the normal fashion,
connecting with the right renal vein to form a normal
right-sided suprarenal IVC. The major clinical
significance of this anomaly is the potential for
misdiagnosis as left-sided para-aortic adenopathy. Spontaneous rupture of an abdominal aortic aneurysm
into a left IVC has also been reported.[3]
The presence of venous variations and anomalies can
have a substantial influence on surgical and interventional
procedures. For instance, in cardiothoracic surgery, renal
transplant surgery, transfemoral cardiac or superior vena
cava procedures or internal jugular vein or subclavian
vein catheter placement, they can contribute to life
threatening complications. Transjugular access to the
infrarenal IVC for filter placement may be difficult
and filter efficiency in cases of the double IVC may be
diminished.
The number of patients requiring haemodialysis is
increasing on an annual basis. According to international
guidelines, an arteriovenous fistula or graft should be the
preferred means of vascular access for haemodialysis.[4] They have good long-term patency and a low rate of
infectious and thrombotic complications. Despite the
recommendations, the number of haemodialysis patients
using central venous catheters as their principal access
is growing worldwide. In 2016 in the United States,
approximately 80% such patients were starting dialysis
with the catheter and 19% were on long-term dialysis.[5]
Analysis of the subgroup of patients aged >75 years
revealed that 48% were on long-term haemodialysis via
a catheter.[6] However, prolonged use of catheter access
is associated with catheter-specific complications that
can ultimately lead to venous damage and exhaustion
of routine venous access via the jugular or subclavian
veins. Femoral access is associated with more frequent
infectious and thrombotic complications and alternative
venous access is then required. With the number of
patients with a central venous haemodialysis catheter
growing, the problem posed by difficult vascular access
is likely to increase.
Although a translumbar direct approach to the IVC
was first described in 1971, the rarity of this procedure
dictates that only relatively small cohorts of patients
have been described.[7] [8] The technique has now been
standardised and most centres perform IVC cannulation
under fluoroscopic control. Puncture is performed from
above the right iliac crest and centred towards the L2
vertebral body, not crossing the midline. Some centres
use a catheter or wire inserted into the IVC beforehand
from the groin puncture to mark the IVC course.
However, in patients with bilateral iliac vein thrombosis it is not possible to insert the wire or catheter into the
IVC. In addition, because of the lack of accessible veins,
good-quality IVC imaging usually cannot be performed.
Because venous variations can influence the translumbar
catheter insertion substantially, the need for thorough
preprocedural cross-sectional imaging cannot be
overemphasised. The IVC puncture is performed with the
patient in a prone position, so the patient should also be
in a prone position for the preprocedural CT or magnetic
resonance imaging examination. This avoids the risk of
variations in anatomy caused by organ movement when
the patient is scanned in a traditionally supine position.
Venous phase with good opacification of the venous
system should be obtained whenever possible.
Data on CT and cone-beam CT navigated procedures
instead of fluoroscopy have been recently published.[9] [10]
In general, and in patients with limited access, these two
methods allow for exact pathway planning and direct
puncture needle visualisation. The drawback of CT
navigation is the need for a two-step hybrid procedure
and transfer of the patient between two examination
rooms. This problem can be solved by C-arm navigation
performed in the same CT examination room. The cone-beam
CT provides three-dimensional data acquired by
detector rotation in the angiography suite. Dedicated
software for needle navigation can also be used. In this
way the procedure can be performed in one location,
combining the advantages of cross-sectional and
fluoroscopic imaging. In our institution we routinely use
CT-navigated IVC cannulation that limits the potential
for complications. Upon successful IVC puncture the
patient is transferred next door to the angiography suite
for TLC placement. It should be noted though that three-dimensional
navigation procedures increase the radiation
dose considerably relative to fluoroscopy.
Without a thorough knowledge of venous anatomy, TLC
insertion can be complicated at several stages. During
IVC cannulation, there may be inadvertent puncture of
the aorta, bowel, kidney, ureter, duodenum or spleen.
One must keep in mind that the puncture track is later
dilated to accommodate a 16F peel away sheath and
this may cause substantial damage to nearby structures.
Insertion of the sheath and catheter into the left IVC is
more risky because of the angulations between the left
IVC, left renal vein and normally positioned right-sided
suprarenal segment of IVC, all of which can be injured
more easily during wire, dilator, sheath and catheter
manipulations. Catheter malposition or kinking may lead
to insufficient function or even vessel wall perforation.
In the long-term, indwelling catheters with more curves
can induce fibrosis and vein thrombosis more often than
in the normal straight course of the right-sided IVC.
With other types of venous variations, an interventional
radiologist can face similar problems due to the small
diameter of the veins, changes in diameter and irregular
course of the veins compared with normal anatomy.
Our patient was transferred to our institution for TLC
placement. No previous cross-sectional examination
or medical history of IVC variation was available at
the time of admission. Early recognition of the left
IVC during CT navigation allowed for change of the
puncture site, safe puncture, and subsequent tunnelled
catheter placement. No early complications were noted,
and the catheter functioned well for 29 months until the
patient died of myocardial infarction. To the best of our
knowledge this is the first publication of TLC insertion
into the left IVC.
It is crucial to diagnose and describe IVC anomalies
and variations to enable proper planning of surgical and
interventional procedures. Lack of awareness of these
anomalies may lead to severe and potentially deadly
complications.
REFERENCES
1. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr.
Spectrum of congenital anomalies of the inferior vena cava: cross-sectional
imaging findings. Radiographics. 2000;20:639-52. Crossref
2. Petik B. Inferior vena cava anomalies and variations: imaging and
rare clinical findings. Insights Imaging. 2015;6:631-9. Crossref
3. Sheth S, Fishman EK. Imaging of the inferior vena cava with
MDCT. AJR Am J Roentgenol. 2007;189:1243-51. Crossref
4. National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis.
2015;66:884-930. Crossref
5. The United States Renal Data System. 2018 ADR Chapters.
Available from: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed 23 Mar 2020.
6. Arhuidese IJ, Cooper MA, Rizwan M, Nejim B, Malas MB. Vascular access for hemodialysis in the elderly. J Vasc Surg.
2019;69:517-25.e1. Crossref
7. Lund GB, Trerotola SO, Scheel PJ Jr. Percutaneous translumbar
inferior vena cava cannulations for hemodialysis. Am J Kidney
Dis. 1995;25:732-7. Crossref
8. Power A, Singh S, Ashby D, Hamady M, Moser S, Gedroyc W, et al.
Translumbar central venous catheters for long-term haemodialysis.
Nephrol Dial Transplant. 2010;25:1588-95. Crossref
9. Kariya S, Tanigawa N, Kojima H, Komemushi A, Shomura Y,
Ha-Kawa SK, et al. Percutaneous translumbar inferior vena cava
cannulation under computed tomography guidance. Jpn J Radiol.
2009;27:176-9. Crossref
10. Thakor AS, Chung J, Patel R, Cormack R, Legiehn, Klass D. The
use of cone-beam CT in assisting percutaneous translumbar catheter
placement into the inferior vena cava. Clin Radiol. 2015;70:21-4. Crossref