Anti-N-methyl-D-aspartate Receptor Encephalitis Magnetic Resonance Imaging and Clinical Features: A Case Series
ORIGINAL ARTICLE
Anti-N-methyl-D-aspartate Receptor Encephalitis Magnetic Resonance Imaging and Clinical Features: A Case Series
CL Chiu1, KH Wong1, MH Lai2, YM Lai1
1 Department of Radiology, North District Hospital, Alice Ho Miu Ling Nethersole Hospital, Hong Kong
2 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong
Correspondence: Dr CL Chiu, Department of Radiology, North District Hospital, Alice Ho Miu Ling Nethersole Hospital, Hong Kong. Email: gabby.chiu@gmail.com
Submitted: 24 Sep 2020; Accepted: 7 Dec 2020.
Contributors: CLC and KHW designed the study. CLC acquired the data. All authors analysed the data. CLC drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref 2020.428). The requirement of informed consent for the study was waived. All clinical investigations were conducted in
accordance with the principles expressed in the Declaration of Helsinki. All patient records were anonymised prior to analysis.
Abstract
Introduction
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a neuro-immunological disease
presenting with a variety of neuropsychiatric features and magnetic resonance imaging (MRI) findings. This case
series investigates the patterns of MRI features of these patients and their possible correlation with clinical parameters.
Methods
Clinical records and brain MRI features of 17 patients diagnosed with anti-NMDAR encephalitis were
reviewed retrospectively. Correlation of imaging features with clinical parameters, including demographics,
presentation, and clinical outcome, was investigated.
Results
Seven patients (41.2%) had abnormal brain MRI findings at presentation. The temporal lobe (excluding
the hippocampus) was the commonest site of involvement, followed by the hippocampus and the insula. Normal
MRI findings at presentation and hippocampal involvement showed no correlation with the clinical parameters.
Interval development of cerebral atrophy (global or localised medial temporal atrophy) on repeat imaging was
associated with worse functional outcome measured on the modified Rankin scale (p = 0.008) and with a lower rate
of symptom-free recovery (p = 0.045).
Conclusion
More than half of our study subjects had normal brain MRI findings at presentation. The temporal lobe
(excluding the hippocampus) was the commonest site of abnormal signal. Interval development of cerebral atrophy
was associated with worse functional outcome and lower rate of symptom-free recovery.
Key Words: Anti-N-methyl-D-aspartate receptor encephalitis, Autoimmune diseases of the nervous system, Magnetic
resonance imaging, Paraneoplastic syndromes, Neuroimmunomodulation
中文摘要
抗N-甲基-D-天冬氨酸受體腦炎的磁共振成像和臨床特徵:病例系列
招卓倫、黃健開、賴銘曦、黎仰文
引言
抗-N-甲基-D-天冬氨酸受體(抗NMDAR)腦炎是一種神經免疫疾病,具有多種神經精神和磁共振成像(MRI)表現。本病例系列研究該病患者的MRI表現及其與臨床參數的可能相關性。
方法
回顧分析17例抗NMDAR腦炎患者的臨床記錄和腦部MRI表現,並研究影像表現與臨床參數的相關性,包括人口統計學、起病表現和臨床結果。
結果
7例(41.2%)就診時腦部MRI結果異常。顳葉(不包括海馬體)是最常見的受累部位,其次是海馬體和島葉。就診時MRI結果正常和海馬體受累顯示與臨床參數無關。一段時間內重複成像顯示發生腦萎縮(整體或局部內側顳葉萎縮)與改良版Rankin量表測量出的較差功能結果(p = 0.008)及較低無症狀康復率(p = 0.045)相關。
結論
超過一半研究對象在就診時的腦部MRI結果正常。 顳葉(不包括海馬體)是異常信號的最常見部位。一段時間內發生腦萎縮與功能較差的結果及較低無症狀康復率相關。
INTRODUCTION
N-methyl-D-aspartate (NMDA) receptors are
heteromeric receptors for glutamate and glycine, which
bind to receptor subunits (one NR1 and multiple NR2
subunits) for activation of NMDA receptors and their
downstream cascade.[1] They play crucial roles in synaptic
transmission as well as in processes such as dendritic
sprouting, synaptic modification, and control of gene
expression.[2]
Disturbance of NMDA receptor activity is associated
with neuropsychiatric abnormalities, including cognitive
impairment and physiologic abnormalities observed in
schizophrenia; as well as with excitotoxicity implicated
in disorders including epilepsy, Parkinson’s disease, and
Huntington’s disease.[2] [3]
Anti-NMDA receptor (anti-NMDAR) encephalitis is a
disease associated with autoantibodies generated against
the NMDA receptors. It was first described by Dalmau et
al in 2007 in women with ovarian teratomas presenting
with psychiatric and neurological symptoms, who
were all noted to have antibodies reacting with NMDA
receptors.[4]
Subsequent studies and case reports have shown that
the condition can affect all ages and both sexes, with or
without associated neoplasm.[5] [6] [7]
A broad spectrum of symptomatology has been
described, ranging from a non-specific flu-like
prodrome (which could include fever, upper respiratory
symptoms and gastrointestinal disturbance), cognitive
disturbance (amnesia, impaired short-term memory),
psychiatric symptoms (confusion, delusions and
hallucinations), neurological symptoms (dyskinesia,
convulsions), to life-threatening conditions such as
coma, autonomic nervous system instability, or status
epilepticus.[8] [9] [10] [11] [12]
Larger-scale studies that focused on the imaging aspects
of anti-NMDAR encephalitis have had some limitations.
For example, brain imaging findings in patients have
been noted to be highly variable in previous case
reports and studies.[13] [14] Normal findings on brain
magnetic resonance imaging (MRI) at presentation are
not uncommon in patients with the disease and, when
there are abnormalities, any parts of the brain can be
affected.[13] [14] [15] [16]
Recent studies have investigated the imaging features
in advanced imaging protocols (including functional
connectivity, diffusion tensor imaging, and voxel-based
morphometry), looking for correlations between the
imaging features and clinical parameters of the disease,
to establish the role of imaging in this relatively new
disease entity.[16] [17] [18]
The aim of this study was to review the imaging features
and clinical parameters of patients with anti-NMDAR encephalitis.
METHODS
Patient
Patients were identified from electronic patient records,
including clinical data analysis and reporting system and
the radiology information system, from three regional
hospitals in Hong Kong. Search criteria were the
primary ICD-9/10 diagnostic code corresponding to anti-NMDAR encephalitis and keywords of imaging requests
with ‘NMDA’. Inclusion criteria were patients admitted
between 2005 and 2020; testing positive for anti-NMDA
receptor antibodies (in either serum or cerebrospinal
fluid); with brain MRI obtained at presentation; and with
images available for review.
Data Collection
The electronic records of each included case were
reviewed. The collection of patient information,
including epidemiological and demographic variables
(age, sex, clinical presentation), cerebrospinal fluid and
serum analysis, imaging (MRI and, where appropriate,
computed tomography and ultrasound), treatment, and
clinical progress/outcome, was performed by manual
review of electronic patient records. The functional
outcome of each patient was graded on a modified
Rankin scale according to the most recent follow-up
clinical data.
Brain Magnetic Resonance Imaging
The multicentre nature of the study and the various
clinical setups did not allow standardisation of sequences.
Images were acquired on different scanners, including
Philips Achieva 1.5 T, Philips Ingenia 1.5 T, Philips
Achieva TX 3 T (Philips, Inc. Best, the Netherlands) and
GE Signa Architect 3 T (GE Healthcare, Inc., Milwaukee
[WI], United States).
The most frequently performed sequences were
T1-weighted spin-echo with and without contrast
enhancement, T2-weighted spin-echo, diffusion-weighted
imaging (DWI), T2-weighted gradient-echo
or susceptibility-weighted imaging, and fluid-attenuated
inversion recovery (FLAIR).
Interpretation of Magnetic Resonance Imaging
All MRIs of the included patients, including those performed at presentation and, if any, those performed
at subsequent reassessment, was reviewed after
anonymisation by two experienced radiologists
independently (both with >12 years of experience in
neuroradiology).
Statistical Analysis
Imaging features, presenting symptoms, associated
clinical conditions, and clinical progress are described
with descriptive statistics (Tables 1 and 2).
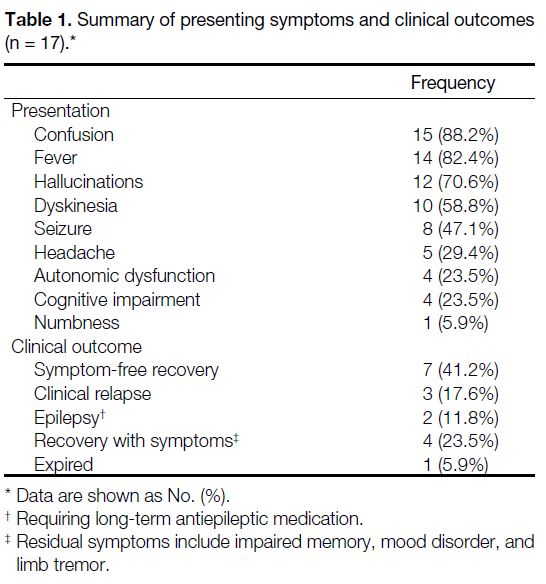
Table 1. Summary of presenting symptoms and clinical outcomes (n = 17).
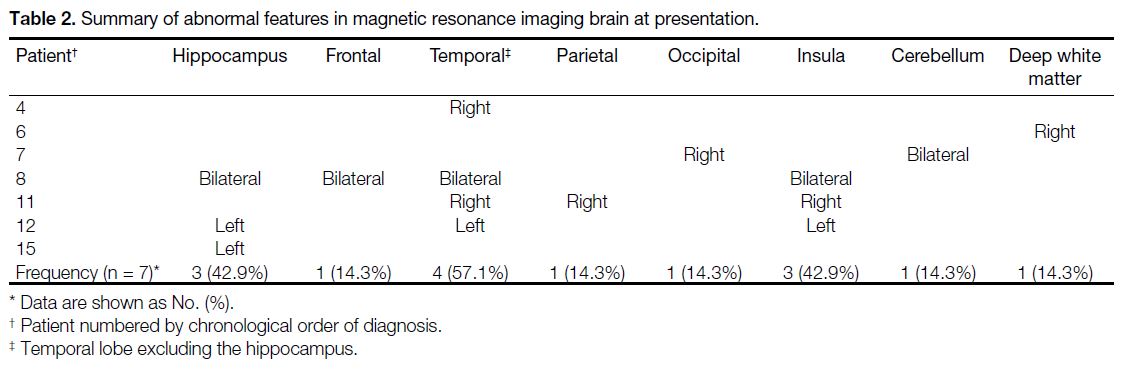
Table 2. Summary of abnormal features in magnetic resonance imaging brain at presentation.
Inferential statistical analysis was performed by
comparing the different qualitative variables, including
brain MRI features and clinical parameters including
demographics, presentation, and clinical progress.
Independent variables including imaging features
and clinical symptoms are categorised using methods
employed in existing literature. Inferential statistical
analysis was accomplished with Fisher’s exact test for
categorical variables, and the Mann-Whitney U test for
ordinal and continuous variables.
The statistical analysis was performed using commercial
software (SPSS Windows version 24.0; IBM Corp,
Armonk [NY], United States). Results with p value
≤0.05 were considered as statistically significant.
RESULTS
Demographics and Clinical Parameters
A total of 19 patients, who tested positive for anti-NMDA receptor antibodies either in serum or cerebrospinal
fluid, were identified. Two of these patients were
excluded, one due to simultaneous herpes simplex virus
encephalitis, and the other because no brain imaging had
been performed. Finally, 17 patients were included, with
median age at diagnosis 29 years (range, 5-42 years;
2 male, 15 female). Two were paediatric patients, aged 5
and 12 years (Table 1).
Four female patients (23.5%) were found to have
neoplasms, all of which were ovarian teratomas
(3 mature teratomas and 1 immature teratoma). All four
underwent subsequent surgical resection of the tumours.
Sixteen patients (94.1%) received immunotherapy,
with steroid and intravenous immunoglobulin being the
commonest agents employed. One patient (5.9%) refused
immunotherapy and was given only antipsychotic and
antidepression medications.
Three patients (17.6%) had at least one episode of clinical relapse after recovery from the presenting symptoms,
which included two patients with seizure and fever and
one with acute delirium and hallucinations. Two patients
(11.8%) were noted to have ongoing epilepsy requiring
long-term antiepileptic medication.
Imaging Features
Signal anomalies are defined as hyperintense signal
in T2-weighted sequences, with or without restricted
diffusion and post-gadolinium enhancement.
Ten (58.8%) of the seventeen patients were noted to
have normal brain MRI findings at presentation, with
a spectrum of abnormalities in the other seven (41.2%)
patients (Table 2, Figures 1 and 2).
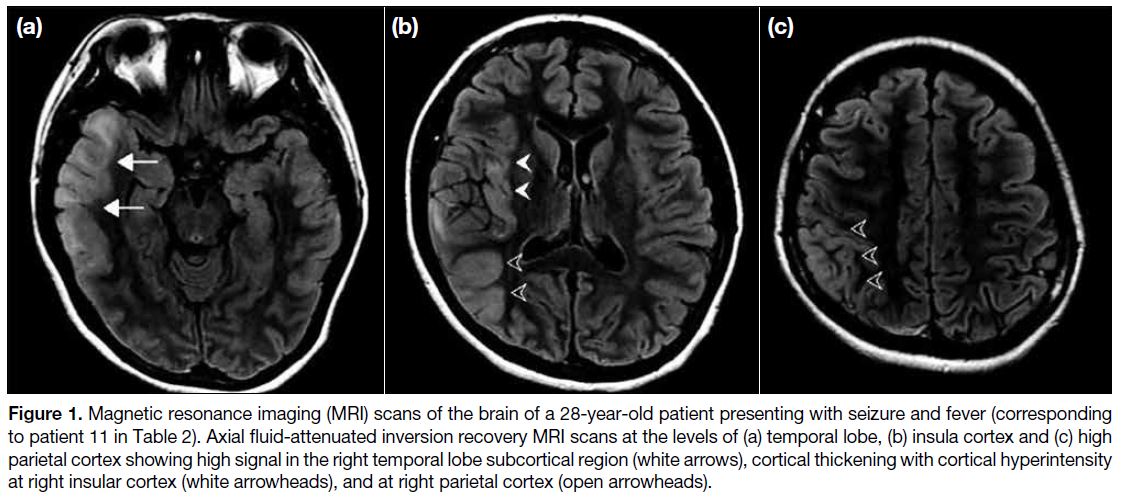
Figure 1. Magnetic resonance imaging (MRI) scans of the brain of a 28-year-old patient presenting with seizure and fever (corresponding
to patient 11 in Table 2). Axial fluid-attenuated inversion recovery MRI scans at the levels of (a) temporal lobe, (b) insula cortex and (c) high
parietal cortex showing high signal in the right temporal lobe subcortical region (white arrows), cortical thickening with cortical hyperintensity
at right insular cortex (white arrowheads), and at right parietal cortex (open arrowheads).
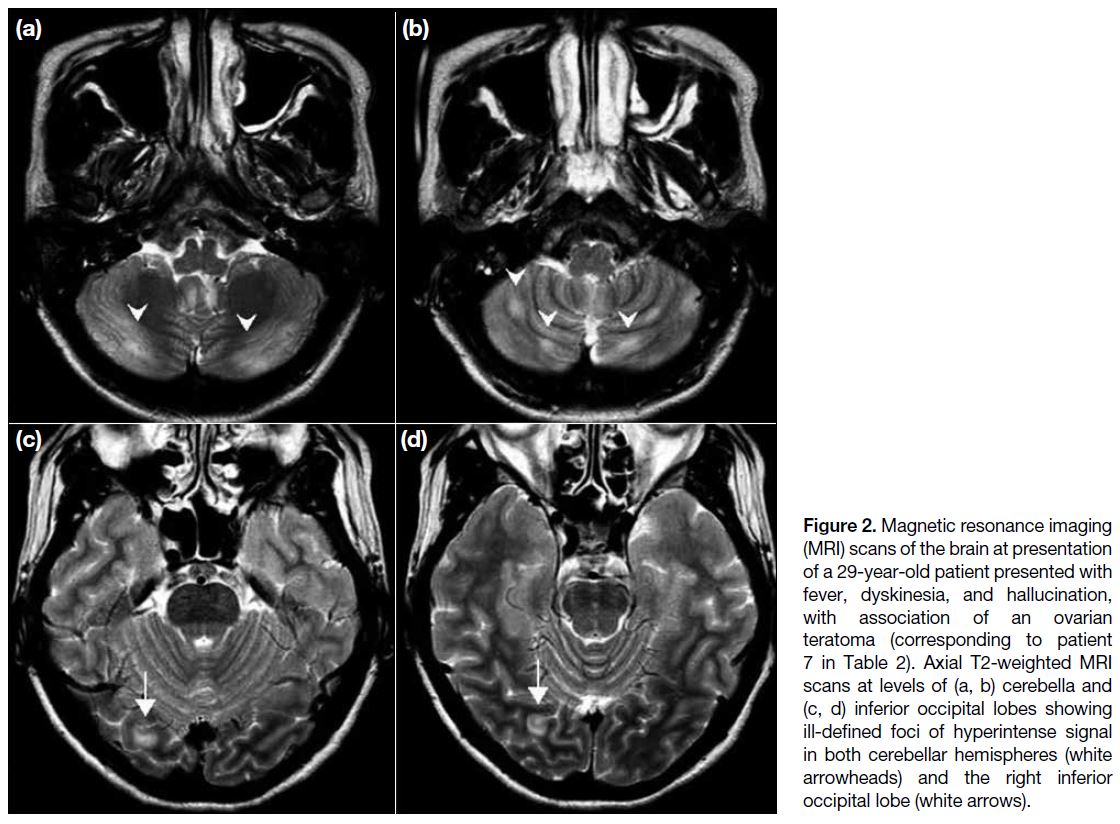
Figure 2. Magnetic resonance imaging
(MRI) scans of the brain at presentation
of a 29-year-old patient presented with
fever, dyskinesia, and hallucination,
with association of an ovarian
teratoma (corresponding to patient
7 in Table 2). Axial T2-weighted MRI
scans at levels of (a, b) cerebella and
(c, d) inferior occipital lobes showing
ill-defined foci of hyperintense signal
in both cerebellar hemispheres (white
arrowheads) and the right inferior
occipital lobe (white arrows).
Of the seven patients with abnormal MRI brain at time
of presentation, the most frequently affected locations were the temporal lobe excluding the hippocampus (n=4,
57.1%), followed by the hippocampus (n=3, 42.9%)
and insula (n=3, 42.9%). Four (57.1%) patients showed
lesions in more than one location. There was only one
(14.3%) patient in whom the hippocampus was the only
location that showed abnormal signal.
Eleven out of the 17 patients (65%) had repeat MRI
examinations at least 2 months after clinical recovery
from initial presentation. Five of these 11 patients had
normal MRI at time of presentation, whereas six had
abnormal MRI. The median time interval to the first repeat
MRI since hospital discharge was 18 months (range, 2.5-180 months). Five of the 11 patients had normal scans,
either from resolution of previous abnormal findings or
remaining normal since presentation. Of the other six
patients with abnormal repeat MRIs, three showed new
locations of abnormality, whereas five showed interval
development of cerebral atrophy (3 patients with global
atrophy and 2 with localised medial temporal lobe
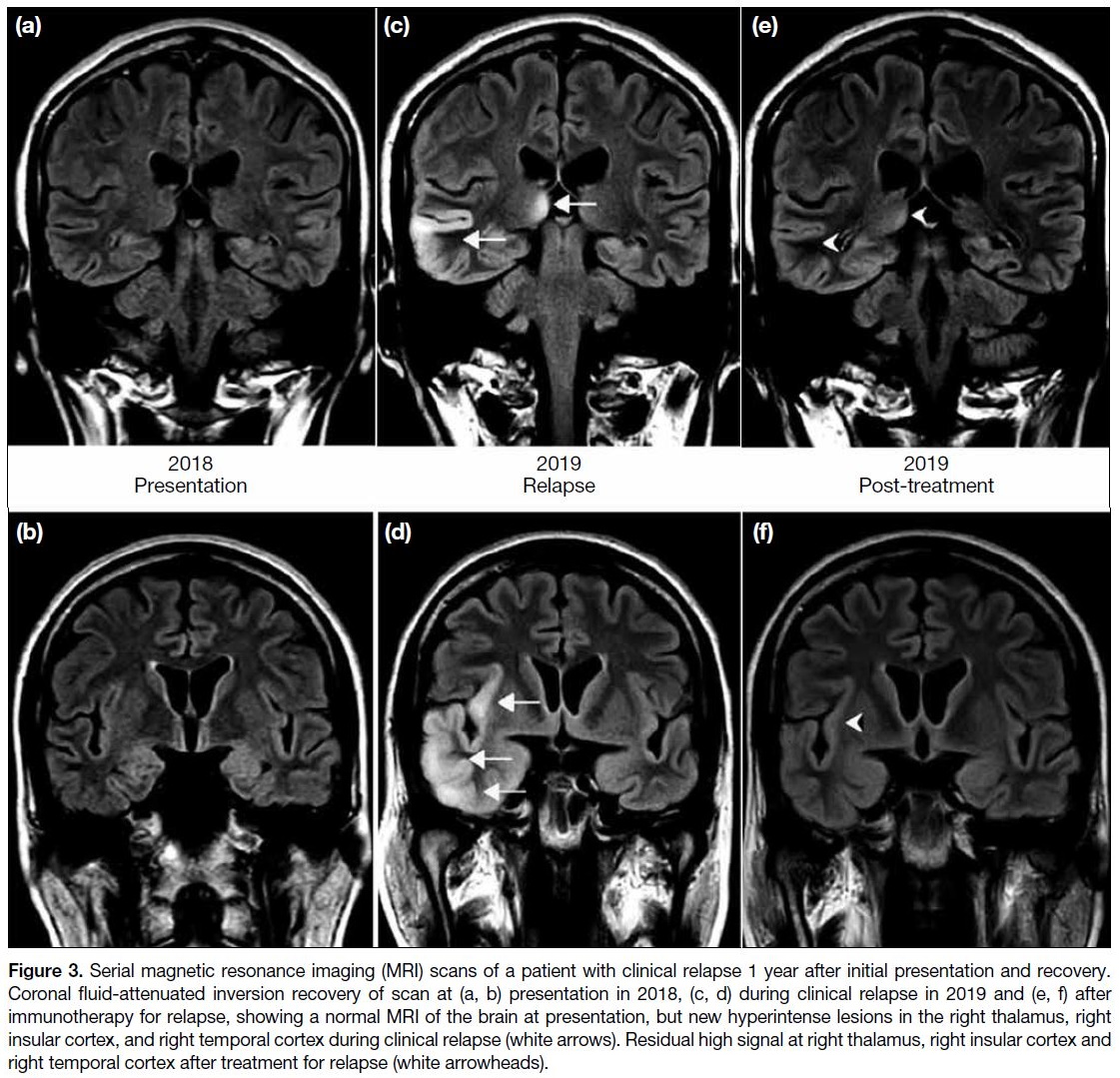
atrophy). Figure 3 shows a patient with a normal MRI at presentation, who developed multiple abnormal findings
on repeat MRI during a clinical relapse.
Figure 3. Serial magnetic resonance imaging (MRI) scans of a patient with clinical relapse 1 year after initial presentation and recovery.
Coronal fluid-attenuated inversion recovery of scan at (a, b) presentation in 2018, (c, d) during clinical relapse in 2019 and (e, f) after
immunotherapy for relapse, showing a normal MRI of the brain at presentation, but new hyperintense lesions in the right thalamus, right
insular cortex, and right temporal cortex during clinical relapse (white arrows). Residual high signal at right thalamus, right insular cortex and
right temporal cortex after treatment for relapse (white arrowheads).
Among the 11 patients with repeat MRI examinations,
analysis of the imaging features at presentation showed
no significant correlation with subsequent development
of cerebral atrophy at reassessment.
Association between Imaging Features and
Clinical Observations
The relationship between imaging features and clinical
parameters was investigated. The sex and age of our
patients showed no significant correlation with normal
MRI brain at time of presentation, hippocampal
involvement, or development of cerebral atrophy.
Normal MRI at presentation and involvement of the
hippocampus had no significant correlation with any the
clinical parameters considered in our patients, including
presentation, association of tumour/teratoma, serum
antibody results, or clinical outcome.
Among the 11 patients with repeat MRI, interval
development of cerebral atrophy (either global atrophy
or localised medial temporal atrophy) was noted to be
associated with worse functional outcome as evidenced
by a higher modified Rankin scale score (p = 0.008).
Patients who did not show any cerebral atrophy on repeat
imaging had a higher rate of symptom-free recovery
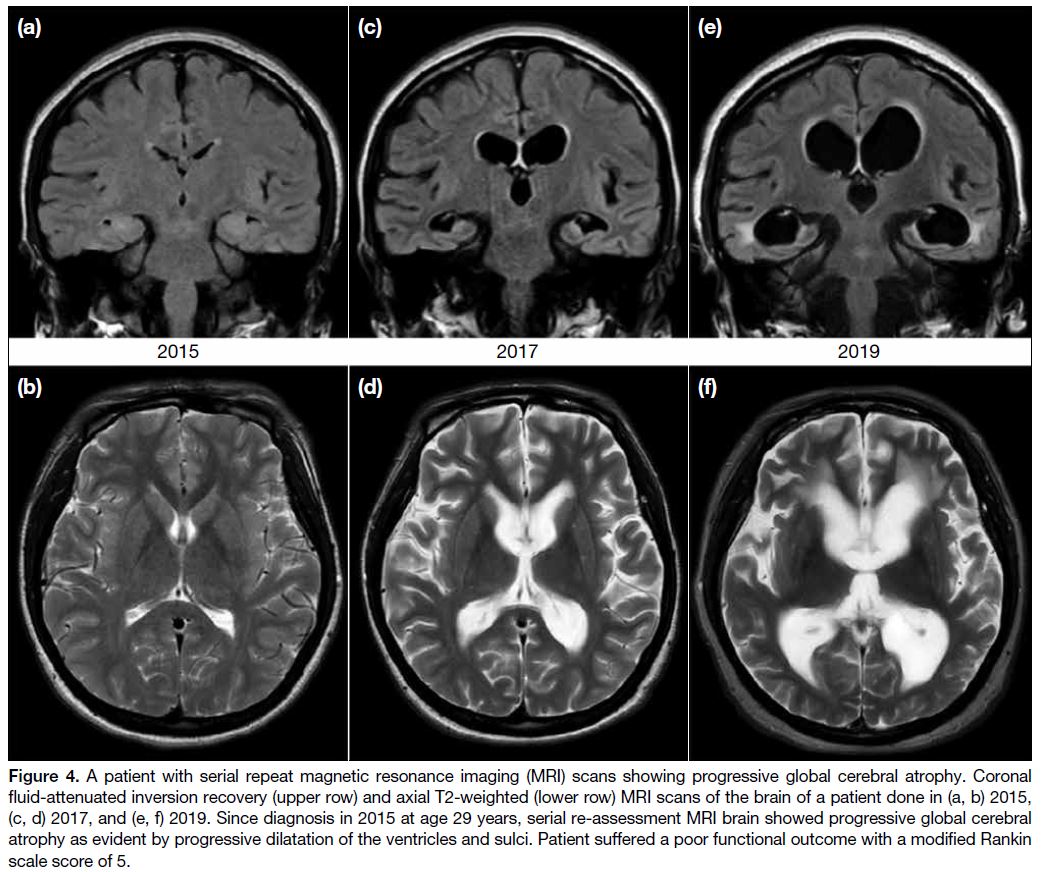
(p = 0.045). Figure 4 features the progressive global
cerebral atrophy observed on serial repeat MRIs of a
patient with a progressively deteriorating clinical course
and poor functional outcome.
Figure 4. A patient with serial repeat magnetic resonance imaging (MRI) scans showing progressive global cerebral atrophy. Coronal
fluid-attenuated inversion recovery (upper row) and axial T2-weighted (lower row) MRI scans of the brain of a patient done in (a, b) 2015,
(c, d) 2017, and (e, f) 2019. Since diagnosis in 2015 at age 29 years, serial re-assessment MRI brain showed progressive global cerebral
atrophy as evident by progressive dilatation of the ventricles and sulci. Patient suffered a poor functional outcome with a modified Rankin
scale score of 5.
DISCUSSION
Anti-NMDAR encephalitis has been noted to have variable
and nonspecific MRI brain features including locations
of involvement and signal characteristics.[13] [14] Common
involvement of the temporal lobe and hippocampus
renders viral encephalitis (particularly herpes simplex
virus), and other autoimmune paraneoplastic encephalitis
high on the list of radiological differential diagnosis.
Extrahippocampal involvement of vascular territories
with restricted diffusion should also raise suspicion of
an acute ischaemic event, whereas thalamic and basal ganglia involvement could mimic Japanese encephalitis,
metabolic/toxic encephalopathy, or and Creutzfeldt–Jakob disease, which are important diagnoses to exclude.
Clinical information including history and presentation
play an important role in diagnosis.
The age (range, 5-42 years) and sex (88% female) of
our patients are similar to those in previous studies.[6] [13] [15]
The rate of associated neoplasm varied greatly among
previous studies (10%-60%); among our 17 patients,
four (24%) had ovarian teratomas.[15] [16]
Slightly more than 50% of our patients showed normal
MRI findings at presentation, which is comparable
to previous studies. However, in the seven patients
presenting with abnormal MRI brain findings, the most commonly affected locations were the temporal
lobe (excluding the hippocampus), followed by the
hippocampus, and the insula. Only two of our patients
had multifocal extrahippocampal lesions at presentation
(Figures 1 and 2). This finding is different from previous
studies in which the hippocampus was most commonly
affected.[15] [16] The small sample size of this study does not
allow any conclusions to be made of this finding.
Although cerebral atrophy in repeat scans correlated
with a worse functional outcome, as well as a lower
rate of symptom-free recovery, not all of our patients
had a follow-up MRI for a comprehensive and unbiased
assessment. Indications and time interval for repeat
imaging were also not standardised due to the retrospective
nature of this study. Some patients had second MRIs due to a clinical relapse, whereas some were completely
asymptomatic at time of repeat scan. The time of repeat
imaging ranged from as soon as 2 months after clinical
recovery to as late as 2 years. Although cerebral atrophy
was unlikely to be an acute process as delineated in the
patient shown in Figure 4, there is reasonable suspicion
of underestimation of the incidence of cerebral atrophy
in this study given the short time interval of some follow-up
scans. Cerebral and medial temporal atrophy were
correlated with poorer clinical outcome and disease
severity in previous studies.[15] [16] The lack of correlation
between abnormalities on the MRI at presentation and
subsequent development of cerebral atrophy in repeat
imaging implies that they are nonspecific. However,
it could partly be attributed to a non-standardised
analysis due to the intrinsic heterogeneity in MRI signal
characteristics of multifocal lesions. Standardisation of
repeat imaging schedules, quantitative assessment of
the degree of cerebral atrophy, and subgroup analysis
(global versus localised atrophy) may also shed light
on the actual pathological process that determines the
clinical outcome of the disease.
Despite extensive search for anti-NMDAR encephalitis
patients in this multicentre study, only 19 patients were
noted to be diagnosed with the disease since 2007, the
year of the first description of the illness.[4] Comparing
to a previous study of a similar ethnic population,[16] and
with reference to data in a previous large-scale study,[6]
there is a suggestion of underdiagnosis of the disease
in this locality. Although the basic demographics of
our patients and most of their clinical features appeared
to be comparable to those of most of the previous
publications,[6] [15] [17] small sample size of this study
limits inferential statistical analysis. Grouping and
categorisation of the highly variable imaging features
of anti-NMDAR encephalitis for subgroup analysis,
which may reveal implication on clinical outcome and
prognosis,[16] was not feasible due to the small sample
size.
A previous study investigating into more advanced MRI
sequences (functional connectivity, diffusion tensor
imaging, and voxel-based morphometry) revealed
altered functional connectivity and white matter changes
in anti-NMDAR encephalitis patients not identified on
routine sequences.[14] [18] This may explain some of the
apparently paradoxical symptomatology in our cases
and suggests the use of these sequences in anti-NMDAR
encephalitis patients.
In conclusion, features of routine MRI sequences
(T1-weighted spin-echo with and without contrast
enhancement, T2-weighted spin-echo, DWI, T2-weighted gradient-echo or susceptibility-weighted
imaging, and FLAIR) of patients with anti-NMDAR
encephalitis are nonspecific, with more than half of our
subjects showing normal findings at presentation. The
temporal lobe (excluding the hippocampus) was the most
commonly involved sites of abnormal MRI signal in our
study. Interval development of cerebral atrophy was
shown to be associated with worse functional outcome
and lower rate of symptom-free recovery. Future
studies of larger sample size, with more advanced MRI
protocols, standardised repeat imaging, and subgroup
analysis would be desirable, but the low incidence of the
disease renders these measures problematic to carry out.
REFERENCES
1. Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB. N-methyl-D-aspartate
receptors: different subunit requirements for binding of
glutamate antagonists, glycine antagonists, and channel-blocking
agents. Mol Pharmacol. 1994;45:540-5.
2. Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease.
Neuroscientist. 2005;11:37-49. Crossref
3. Coyle JT. Glutamate and schizophrenia: beyond the dopamine
hypothesis. Cell Mol Neurobiol. 2006;26:365-84. Crossref
4. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A,
et al. Paraneoplastic anti-N-methyl-D-aspartate receptor
encephalitis associated with ovarian teratoma. Ann Neurol.
2007;61:25-36. Crossref
5. Lim JA, Lee ST, Jung KH, Kim S, Shin JW, Moon J, et al. Anti-N-methyl-d-aspartate receptor encephalitis in Korea: clinical features,
treatment, and outcome. J Clin Neurol. 2014;10:157-61. Crossref
6. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations
in patients with anti-NMDAR encephalitis. Lancet Neurol.
2011;10:63-74. Crossref
7. Khundakji Y, Masri A, Khuri-Bulos N. Anti-NMDA receptor
encephalitis in a toddler: a diagnostic challenge. Int J Pediatr
Adolesc Med. 2018;5:75-7. Crossref
8. Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J,
et al. Anti-NMDA-receptor antibody detected in encephalitis,
schizophrenia, and narcolepsy with psychotic features. BMC
Psychiatry. 2012;12:37. Crossref
9. Pollak TA, McCormack R, Peakman M, Nicholson TR, David AS.
Prevalence of anti-N-methyl-d-aspartate (NMDA) receptor
antibodies in patients with schizophrenia and related psychoses: a
systematic review and meta-analysis. Psychol Med. 2014;44:2475-87. Crossref
10. Mann A, Machado NM, Liu N, Mazin AH, Silver K, Afzal KI.
A multidisciplinary approach to the treatment of anti-NMDA-receptor
antibody encephalitis: a case and review of the literature.
J Neuropsychiatry Clin Neurosci. 2012;24:247-54. Crossref
11. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis,
autoimmunity, and psychosis. Schizophr Res. 2016;176:36-40. Crossref
12. Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2011;231:86-91. Crossref
13. Kaur S, Juneja M, Mishra D, Jain S. Anti-N-methyl-D-aspartate
receptor encephalitis: a case report and review of the literature. J
Pediatr Neurosci. 2014;9:145-7. Crossref
14. Sachs JR, Zapadka ME, Popli GS, Burdette JH. Arterial spin
labeling perfusion imaging demonstrates cerebral hyperperfusion
in anti-NMDAR encephalitis. Radiol Case Rep. 2017;12:833-937. Crossref
15. Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M,
et al. Anti-NMDA-receptor encephalitis: case series and analysis
of the effects of antibodies. Lancet Neurol. 2008;7:1091-8. Crossref
16. Zhang T, Duan Y, Ye J, Xu W, Shu N, Wang C, et al. Brain MRI
characteristics of patients with anti-n-methyl-d-aspartate receptor
encephalitis and their associations with 2-year clinical outcome.
AJNR Am J Neuroradiol. 2018;39:824-9. Crossref
17. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an
observational cohort study. Lancet Neurol. 2013;12:157-65. Crossref
18. Finke C, Kopp UA, Scheel M, Pech LM, Soemmer C, Schlichting J,
et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2013;74:284-96. Crossref