Differential Diagnoses of Axillary Lesions: a Pictorial Essay
PICTORIAL ESSAY
Differential Diagnoses of Axillary Lesions: a Pictorial Essay
TKB Lai, T Wong, CM Chau, WY Fung, RLS Chan, AWT Yung, JKF Ma
Department of Diagnostic and Interventional Radiology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr TKB Lai, Department of Diagnostic and Interventional Radiology, Princess Margaret Hospital, Hong Kong. Email: laiterencekb@gmail.com
Submitted: 11 May 2021; Accepted: 15 Jul 2021.
Contributors: All authors designed the study and acquired the data. TKBL, TW and CMC analysed the data. TKBL drafted the manuscript.
All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the KWC Research Ethics Committee (Ref: KW/EX-20-146(153-04)) and conducted in accordance
with the Declaration of Helsinki.
Declaration: Part of the material from this study was accepted as an e-poster presentation at the 28th Annual Scientific Meeting of Hong Kong College of Radiologists, 14-15 November 2020.
INTRODUCTION
Axillary mass or swelling is a common clinical
presentation that requires imaging investigation. There is
a wide range of differential diagnoses because the axilla
contains both lymph nodes and non-lymphatic tissue
such as accessory breast tissue, skin, fat, muscles, nerves,
blood vessels, and is surrounded by bone. Radiologists
should be familiar with the axillary anatomy and imaging
features of different axillary lesions.
This pictorial essay provides an imaging review and
diagnosis of axillary lesions that arise from different
anatomical structures using different imaging modalities
including ultrasonography (US), mammography, plain
radiography, computed tomography (CT), magnetic
resonance imaging (MRI), and angiography.
ANATOMY
A basic understanding of axillary anatomy is essential
for both lesion detection and differentiation. The axilla
resembles a pyramid.[1] [2] There are pectoralis muscles
anteriorly, and the scapula, latissimus dorsi and teres
major muscles posteriorly.[1] The medial and lateral
boundaries comprise the chest wall and proximal humerus,
respectively.[1] The base is covered by skin, while the apex, which contains neurovascular structures, is bounded by
the first rib, clavicle, and superior scapular border.[1]
LYMPH NODES
Axillary lymph nodes are divided into three levels by the
pectoralis minor muscle. Level I is inferolateral to the
pectoralis minor; level II behind the pectoralis minor;
and level III superomedial to the pectoralis minor. A
complete US examination of these lymph nodes should
scrutinise the entire fatty content from the margin of the
pectoralis muscles to the latissimus dorsi and teres major
muscles and should include the axillary tail.[3]
Differentiating malignant lymph nodes from benign is
no easy task due to the presence of overlapping features.
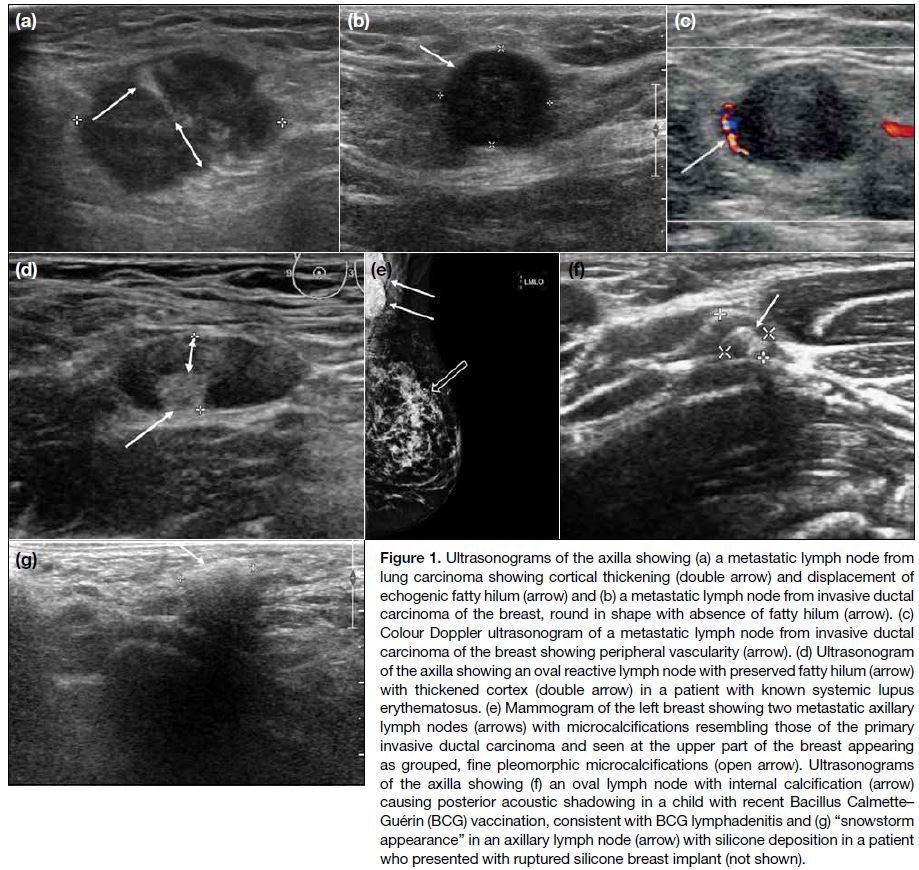
Changes such as cortical thickening (Figure 1a), hilum
loss, or changes in shape or vascular pattern, are
considered suspicious.[4]
Figure 1. Ultrasonograms of the axilla showing (a) a metastatic lymph node from
lung carcinoma showing cortical thickening (double arrow) and displacement of
echogenic fatty hilum (arrow) and (b) a metastatic lymph node from invasive ductal carcinoma of the breast, round in shape with absence of fatty hilum (arrow). (c) Colour Doppler ultrasonogram of a metastatic lymph node from invasive ductal carcinoma of the breast showing peripheral vascularity (arrow). (d) Ultrasonogram of the axilla showing an oval reactive lymph node with preserved fatty hilum (arrow) with thickened cortex (double arrow) in a patient with known systemic lupus erythematosus. (e) Mammogram of the left breast showing two metastatic axillary lymph nodes (arrows) with microcalcifications resembling those of the primary invasive ductal carcinoma and seen at the upper part of the breast appearing as grouped, fine pleomorphic microcalcifications (open arrow). Ultrasonograms of the axilla showing (f) an oval lymph node with internal calcification (arrow) causing posterior acoustic shadowing in a child with recent Bacillus Calmette–Guérin (BCG) vaccination, consistent with BCG lymphadenitis and (g) “snowstorm appearance” in an axillary lymph node (arrow) with silicone deposition in a patient who presented with ruptured silicone breast implant (not shown).
A benign lymph node has a thin or invisible cortex
and a fatty hilum due to connective tissue trabeculae,
lymphatic tissue cords, and medullary sinusoids.[4]
The cortex is hypoechoic on US, while the hilum is
echogenic on US and lucent on mammography. Various
cut-off points have been reported for a normal cortical thickness, with an upper limit ranging from 2.3 mm to
3 mm.[4] Absence of the hilum is the most specific finding
for metastatic disease, but such finding is present only in
cases of advanced disease.[4]
A benign lymph node is usually <2 cm in maximal
diameter.[3] Nonetheless size criteria are reported to be less
essential to identify metastasis.[3] A benign lymph node is
normally oval or elliptical in shape. On the contrary, a
malignant node is often round (Figure 1b) and has a short
to long axes ratio of >0.5.[5]
Nodal vascularity on Doppler US generally follows
two patterns: central or peripheral.[4] The central pattern shows a single hilum vascular signal or dispersed signals
distributed at the centre of the node and is usually found
in the absence of malignancy.[4] The peripheral pattern
(Figure 1c) demonstrates a linear signal at the periphery
of the node and is more common in lymph nodes with
metastases.[4]
Causes of unilateral axillary lymphadenopathy can
be benign and include infection such as mastitis[6] and
post-vaccination reactions, for example from influenza,
human papillomavirus, Bacillus Calmette–Guérin, or
coronavirus disease 2019 vaccination.[7] Malignant causes
include metastasis from breast malignancy and non-breast
malignancies.[6]
Bilateral axillary lymphadenopathy can be due to
systemic aetiologies such as wide-spread infection,
rheumatoid arthritis, collagen vascular disease such as
systemic lupus erythematosus (Figure 1d), lymphoma,
leukaemia, and metastatic non-breast tumour.[6]
Calcifications in abnormal axillary lymph nodes may
represent calcified metastasis from breast (Figure 1e),
thyroid or ovarian cancer, collagen vascular disease,
or granulomatous infections such as tuberculosis and
Bacillus Calmette–Guérin lymphadenitis (Figure 1f).[3]
Migrated silicone from an augmented breast and
migrated gold particles in rheumatoid arthritis patients
receiving gold therapy can mimic calcifications in
lymph nodes, and can usually be differentiated by a
relevant clinical history.[6] Silicone deposition in an
axillary lymph node is typically hyperechoic with a well-defined
anterior margin and a poorly defined posterior
margin, giving a characteristic “snowstorm appearance”
(Figure 1g).
It should be noted that imaging criteria are not completely
reliable in differentiating benign from malignant lymph
nodes. For those with suspicious features, image-guided fine needle aspiration cytology or biopsy can offer
additional information.
Non-nodal Lesions
Knowledge of a relevant clinical presentation such as
prior axillary surgery, breast augmentation or active
local infection can help diagnose certain surgery-related
non-nodal axillary pathologies.
Surgery-Related Lesions
Post-surgical lesions in the axilla include seromas,
haematomas, fat necrosis, and suture granulomas.
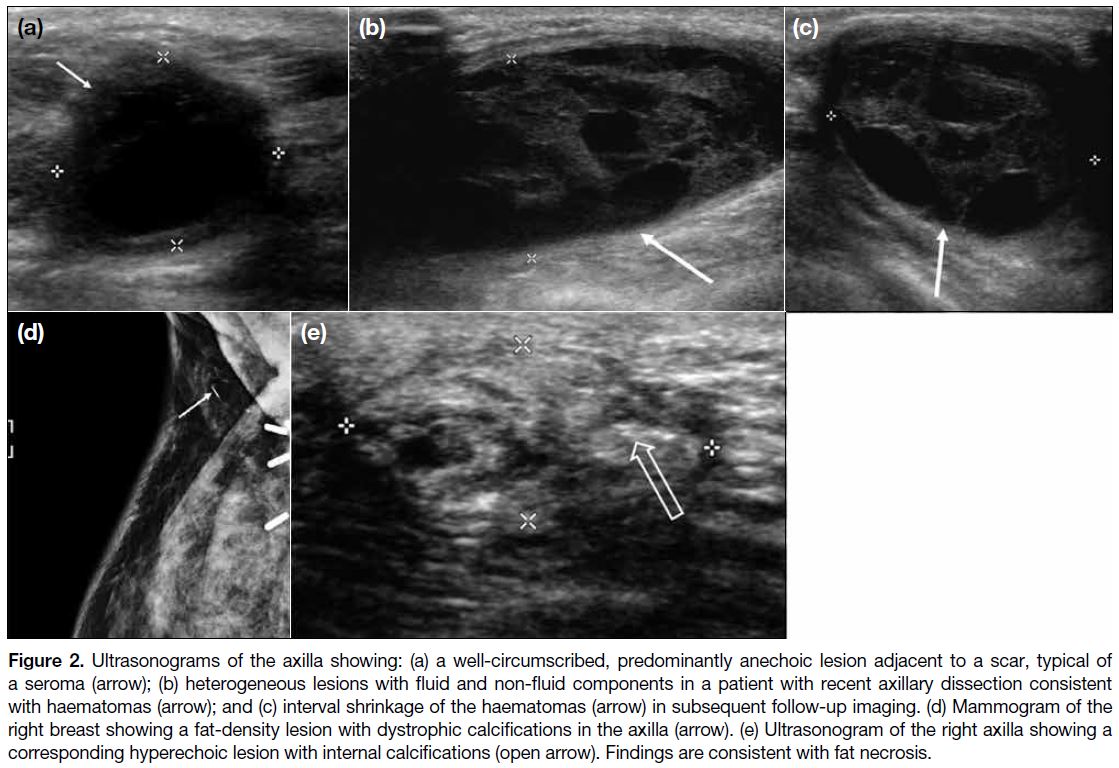
Seromas (Figure 2a) typically appear as loculated fluid
collections close to the operative bed, with or without
fluid-debris levels on US and MRI scans.[8]
Figure 2. Ultrasonograms of the axilla showing: (a) a well-circumscribed, predominantly anechoic lesion adjacent to a scar, typical of
a seroma (arrow); (b) heterogeneous lesions with fluid and non-fluid components in a patient with recent axillary dissection consistent
with haematomas (arrow); and (c) interval shrinkage of the haematomas (arrow) in subsequent follow-up imaging. (d) Mammogram of the
right breast showing a fat-density lesion with dystrophic calcifications in the axilla (arrow). (e) Ultrasonogram of the right axilla showing a
corresponding hyperechoic lesion with internal calcifications (open arrow). Findings are consistent with fat necrosis.
Haematomas may show variable internal architecture
on imaging, depending on the evolution of the blood
products (Figure 2b,c).[8] Appearance ranges from being
anechoic on US during the hyperacute phase, with
progressive development of mixed internal echoes due to
formation of blood clots, to finally becoming lesions with
irregular walls and internal septations in the subacute to
chronic phase.[2]
Fat necrosis is a benign inflammatory process resulting
from vascular insult to fat cells, and its imaging findings
are reported to correspond to the stage of development
and maturation.[8] Appearance on mammography ranges
from a speculated mass to calcifications (Figure 2d,e).
It can appear cystic on US and may contain echogenic
bands that are specific for fat necrosis. Features on
MRI scans correspond to the extent of inflammation
and fibrosis. It often exhibits hypointense signal on
T1-weighted sequences and may demonstrate variable
enhancement in post-contrast images.[2]
Suture granuloma occurs due to local inflammation in
response to retained suture material. It is characterised
by a hypoechoic lesion with hyperechoic lines on US
that represent the sutures.[8]
All these post-surgical lesions may be difficult to
differentiate from malignancy and biopsy is required.[8]
Breast Augmentation–Related Lesions
They are mainly related to migration of ruptured
implants or injected materials. Examples include silicone
deposits in axillary lymph nodes as described previously,
and polyacrylamide gel migration to the axilla as
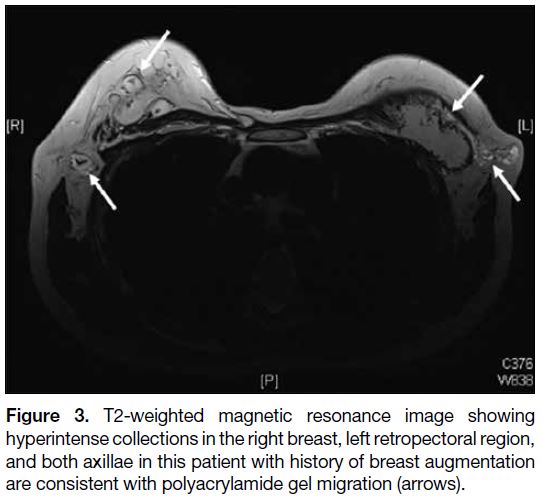
a consequence of breast augmentation (Figure 3).
Migrated polyacrylamide gel appears as small loculations
with MRI signal intensity similar to that of water and can
form nodules in the axilla.[9]
Figure 3. T2-weighted magnetic resonance image showing
hyperintense collections in the right breast, left retropectoral region,
and both axillae in this patient with history of breast augmentation
are consistent with polyacrylamide gel migration (arrows).
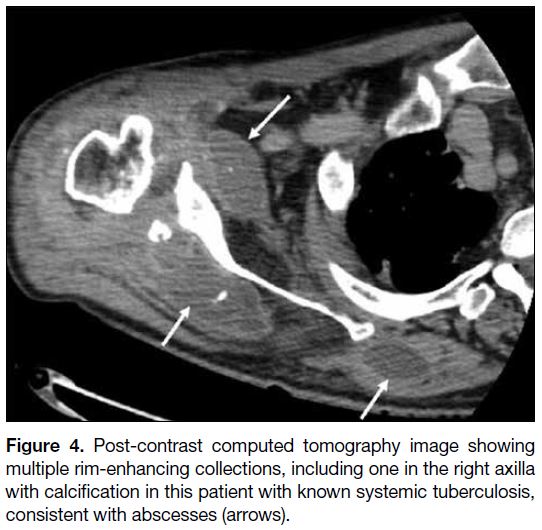
Infective or Inflammatory Lesions
This entity includes abscesses.[1] Local injury, sweat or
sebaceous gland obstruction, and hair follicle infection
can result in abscess formation.[2] Abscesses can also
occur within the lymph nodes as a result of bacterial or
tuberculosis lymphadenitis.[2] On US, they can appear as a hypoechoic fluid collection with internal debris or
loculations and a hypervascular echogenic wall.[2] On
contrast cross-sectional imaging, they usually show
peripheral rim enhancement (Figure 4).
Figure 4. Post-contrast computed tomography image showing multiple rim-enhancing collections, including one in the right axilla with calcification in this patient with known systemic tuberculosis, consistent with abscesses (arrows).
Other non-nodal pathologies can be categorised according
to their anatomical origin and managed accordingly.
BREAST TISSUE
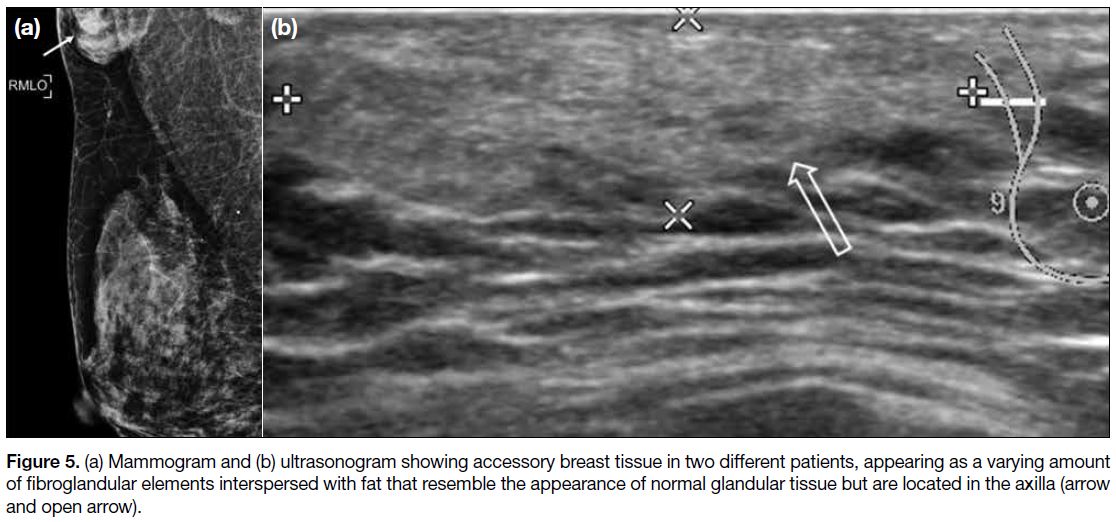
Accessory Breast Tissue
This is a normal variant resulting from failed regression
of embryonic mammary tissue, most often located in
the axilla and is susceptible to the same benign and
malignant pathologies that develop in the normal breast.[3]
On mammography and US, there is a varying amount of fibroglandular elements among fat that radiologically
resembles normal glandular tissue but is separate from
the main breast parenchyma (Figure 5).[2] [3]
Figure 5. (a) Mammogram and (b) ultrasonogram showing accessory breast tissue in two different patients, appearing as a varying amount
of fibroglandular elements interspersed with fat that resemble the appearance of normal glandular tissue but are located in the axilla (arrow
and open arrow).
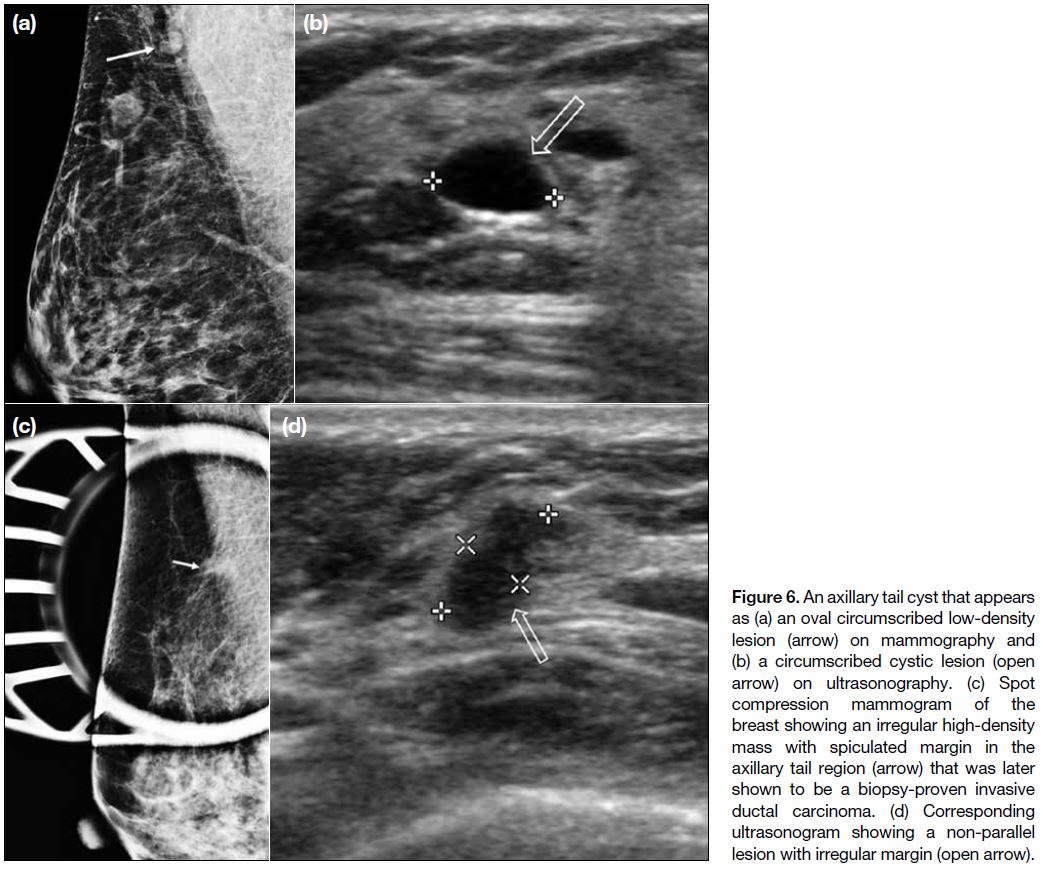
Axillary Tail Lesions
Axillary tail is a continuous extension of the upper outer
quadrant breast tissue. Therefore benign lesions such as
cyst (Figure 6a,b) can develop in the axillary tail as in the
rest of the breast. Breast carcinoma (Figure 6c,d) in the
axillary tail is extremely rare.[10]
Figure 6. An axillary tail cyst that appears as (a) an oval circumscribed low-density lesion (arrow) on mammography and (b) a circumscribed cystic lesion (open arrow) on ultrasonography. (c) Spot compression mammogram of the breast showing an irregular high-density mass with spiculated margin in the axillary tail region (arrow) that was later shown to be a biopsy-proven invasive ductal carcinoma. (d) Corresponding ultrasonogram showing a non-parallel lesion with irregular margin (open arrow).
SKIN
US is the optimal imaging modality to localise superficial
breast lesions.[11] Sonographic features that suggest a
lesion of dermal origin include an acute angle between
the lesion and the dermal line, and a “claw” of skin
wrapping around the margin of a lesion.[11]
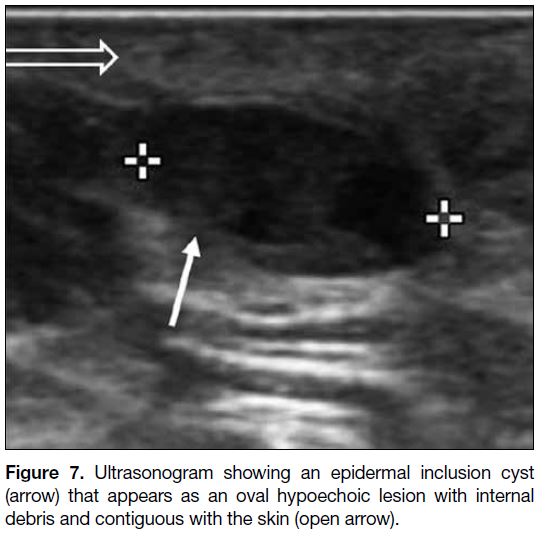
Epidermal Inclusion Cyst
Epidermal inclusion cyst (Figure 7) is a benign dermal
lesion containing keratin and is lined by epidermis.[11] Its
appearance on US varies with its content, ranging from
cystic to hypoechoic or heterogenous.[11] The presence of a tract to the skin, which represents the hair follicle
extending from the dermis up through the epidermis, is
diagnostic.[11]
Figure 7. Ultrasonogram showing an epidermal inclusion cyst (arrow) that appears as an oval hypoechoic lesion with internal debris and contiguous with the skin (open arrow).
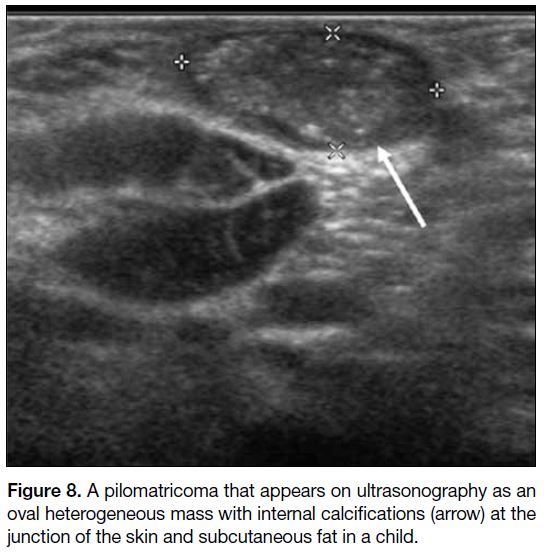
Pilomatricoma
Pilomatricoma is more common in children but
occasionally encountered in adults.[12] It arises from the
lower dermis and extends into the subcutaneous fat as it
grows, with thinning of the overlying dermis.[12] On US
(Figure 8), it appears as a well-defined hypoechoic mass
with echogenic calcific foci, or a completely calcified
mass showing strong posterior acoustic shadow.[12]
Figure 8. A pilomatricoma that appears on ultrasonography as an
oval heterogeneous mass with internal calcifications (arrow) at the
junction of the skin and subcutaneous fat in a child.
SUBCUTANEOUS TISSUES AND MUSCLES
Lipoma
Lipomas often have a homogeneous circumscribed
appearance, some of which may have septations.[2] They
are usually subcutaneous in origin (Figure 9a) but can also arise intramuscularly, including from the pectoralis
muscle in the axilla (Figure 9b), manifesting as a low-density
area on mammography (Figure 9c) and fat density
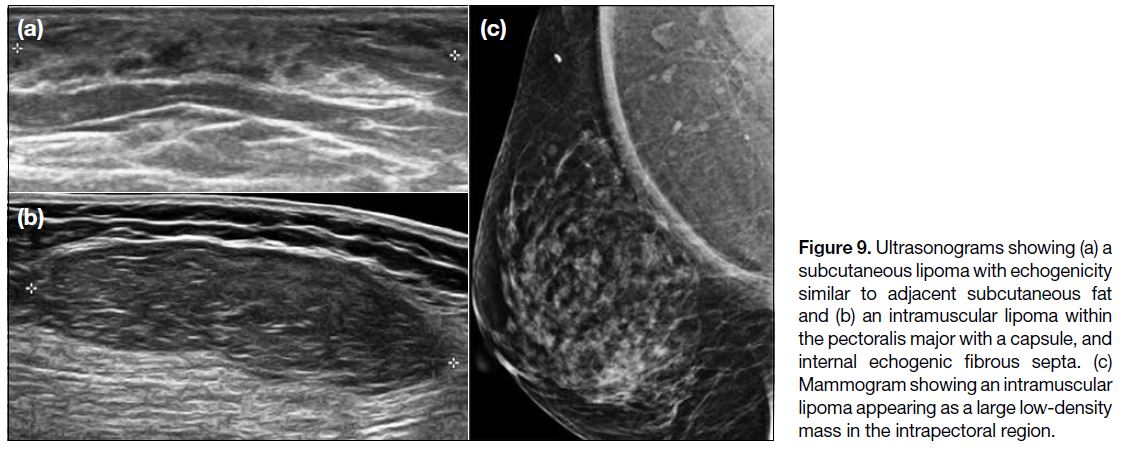
on CT scans.[2] They show variable echogenicity on US and may be capsulated.[2] They typically demonstrate fat signal on MRI scans with minimal contrast enhancement.[2]
Figure 9. Ultrasonograms showing (a) a subcutaneous lipoma with echogenicity similar to adjacent subcutaneous fat and (b) an intramuscular lipoma within the pectoralis major with a capsule, and internal echogenic fibrous septa. (c) Mammogram showing an intramuscular lipoma appearing as a large low-density mass in the intrapectoral region.
Liposarcoma
When a lipomatous lesion shows atypical features
including heterogenicity and contrast enhancement
on cross-sectioned imaging, a liposarcoma should be
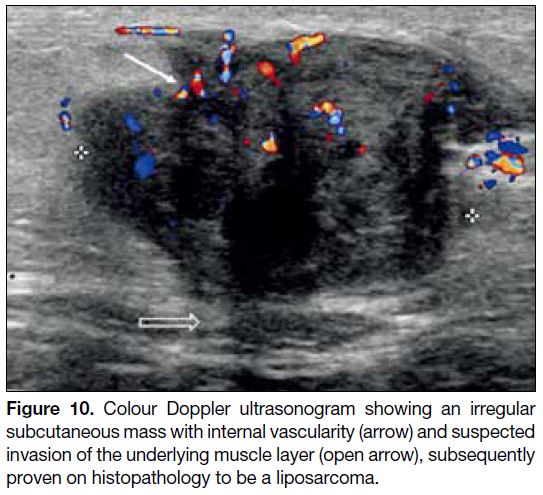
considered.[2] On US (Figure 10), a well-differentiated
liposarcoma appears heterogeneous, multilobulated, and
typically well-defined.[13] Sonographic identification of
hyperechoic fat may be difficult and variable.[13] On CT
and MRI scans, it usually presents as a predominantly
lipomatous mass with non-lipomatous components, most
often as thickened septa with or without nodularity.[13]
Figure 10. Colour Doppler ultrasonogram showing an irregular subcutaneous mass with internal vascularity (arrow) and suspected invasion of the underlying muscle layer (open arrow), subsequently proven on histopathology to be a liposarcoma.
Other Soft Tissue Masses
Other soft tissue masses include other soft tissue sarcomas,
nodular fasciitis, desmoid fibromatosis, and benign
muscular neoplasms such as intramuscular myxoma.[1] [4]
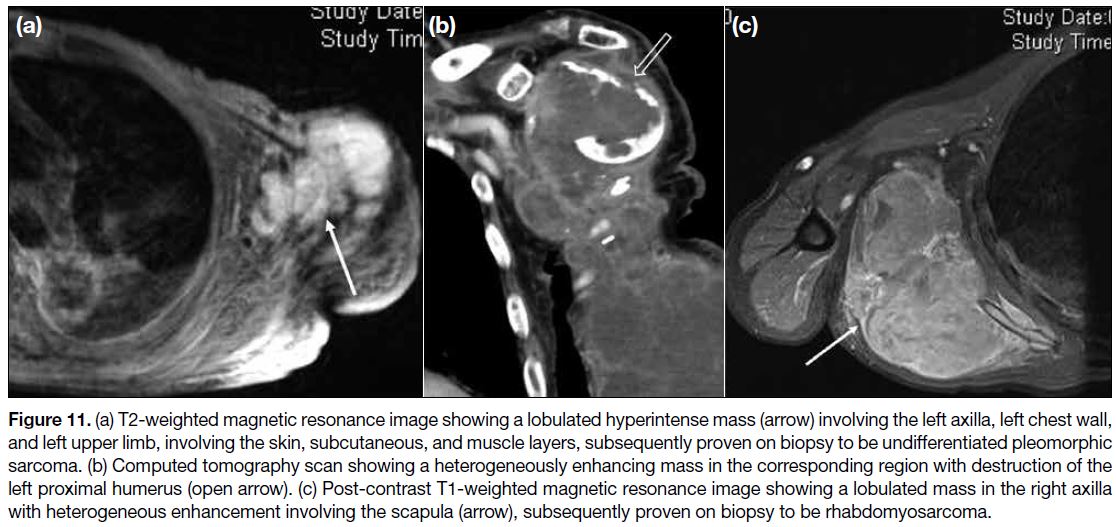
In our centre, we came across undifferentiated
pleomorphic sarcoma (Figure 11a,b) and
rhabdomyosarcoma (Figure 11c) involving the axilla.
Figure 11. (a) T2-weighted magnetic resonance image showing a lobulated hyperintense mass (arrow) involving the left axilla, left chest wall,
and left upper limb, involving the skin, subcutaneous, and muscle layers, subsequently proven on biopsy to be undifferentiated pleomorphic
sarcoma. (b) Computed tomography scan showing a heterogeneously enhancing mass in the corresponding region with destruction of the
left proximal humerus (open arrow). (c) Post-contrast T1-weighted magnetic resonance image showing a lobulated mass in the right axilla
with heterogeneous enhancement involving the scapula (arrow), subsequently proven on biopsy to be rhabdomyosarcoma.
BLOOD VESSELS
Vascular Lesions
Among this heterogeneous group of axillary lesions, vascular malformation and pseudoaneurysms have been
reported.[2] [8]
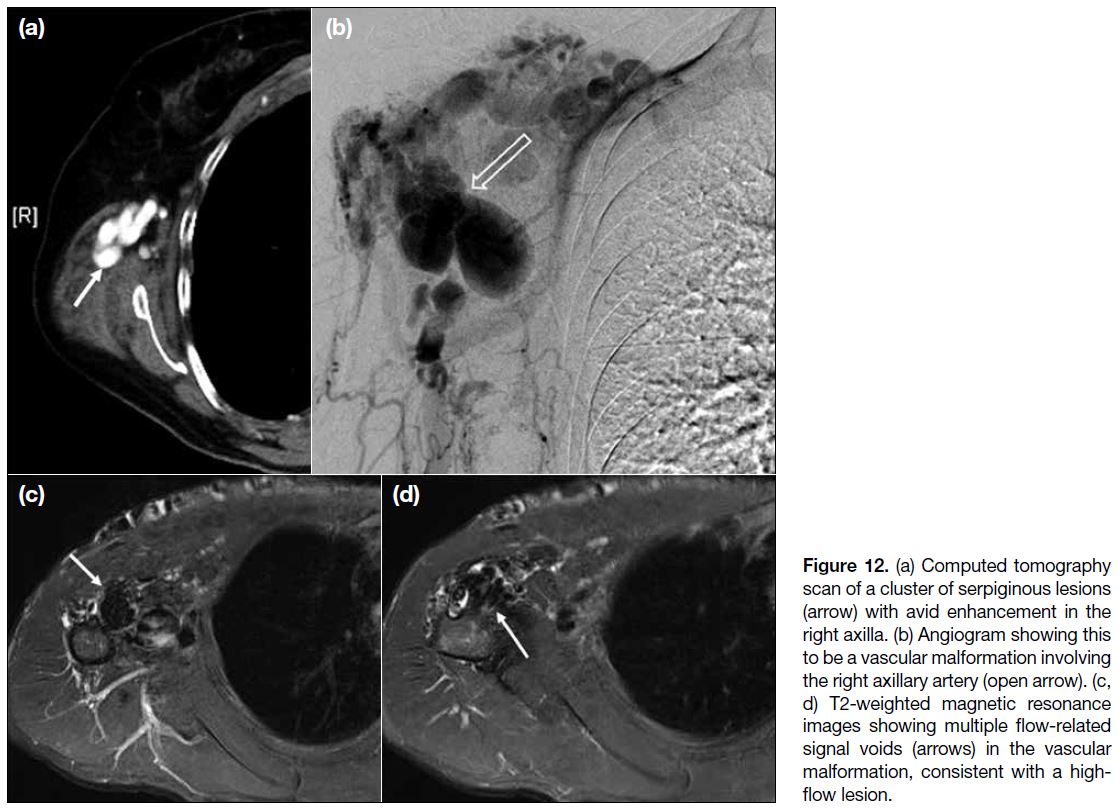
In our centre, we encountered a case of arteriovenous
malformation that manifested as a cluster of enhancing
tortuous lesions on CT scans and angiography
(Figure 12a,b). On MRI scans (Figure 12c,d), signal
voids within the lesions on T2-weighted images indicated
a high-flow component.
Figure 12. (a) Computed tomography scan of a cluster of serpiginous lesions (arrow) with avid enhancement in the right axilla. (b) Angiogram showing this to be a vascular malformation involving the right axillary artery (open arrow). (c, d) T2-weighted magnetic resonance images showing multiple flow-related signal voids (arrows) in the vascular malformation, consistent with a high-flow lesion.
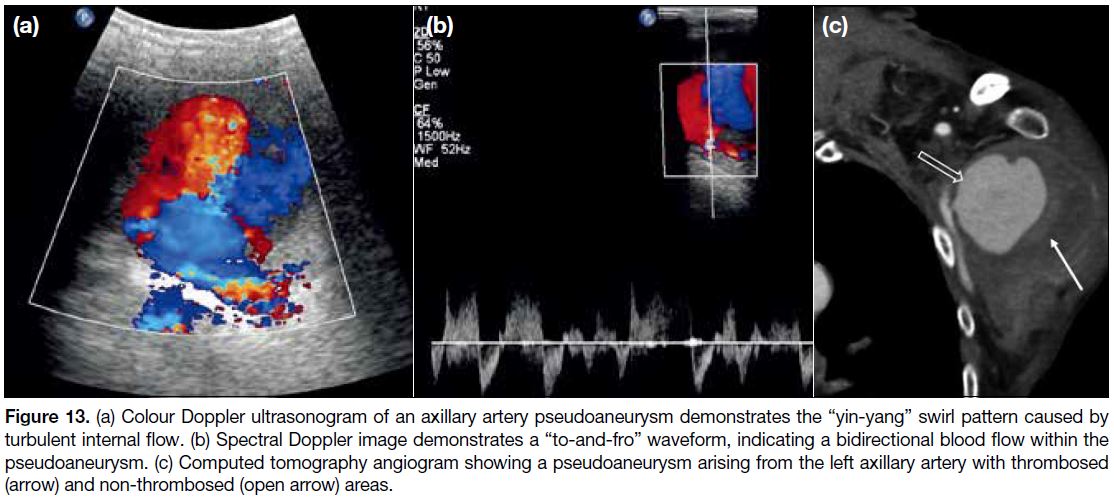
Pseudoaneurysms can be spontaneous or trauma-related.
[14] We encountered a case of axillary artery
pseudoaneurysm secondary to adjacent proximal humeral fracture. On Doppler US, typical findings include
the “yin-yang” sign (Figure 13a) and a “to-and-fro”
waveform on duplex Doppler US (Figure 13b), both of
which are related to turbulent blood flow in the lesion.[14]
Different types of angiography (conventional or CT)
may also help, especially in identifying the donor artery.
Pseudoaneurysm can appear as a low-density lesion on
CT with enhancement similar to the donor artery while
a non-enhanced region within the lesion may signify
thrombosis (Figure 13c).[14]
Figure 13. (a) Colour Doppler ultrasonogram of an axillary artery pseudoaneurysm demonstrates the “yin-yang” swirl pattern caused by
turbulent internal flow. (b) Spectral Doppler image demonstrates a “to-and-fro” waveform, indicating a bidirectional blood flow within the
pseudoaneurysm. (c) Computed tomography angiogram showing a pseudoaneurysm arising from the left axillary artery with thrombosed
(arrow) and non-thrombosed (open arrow) areas.
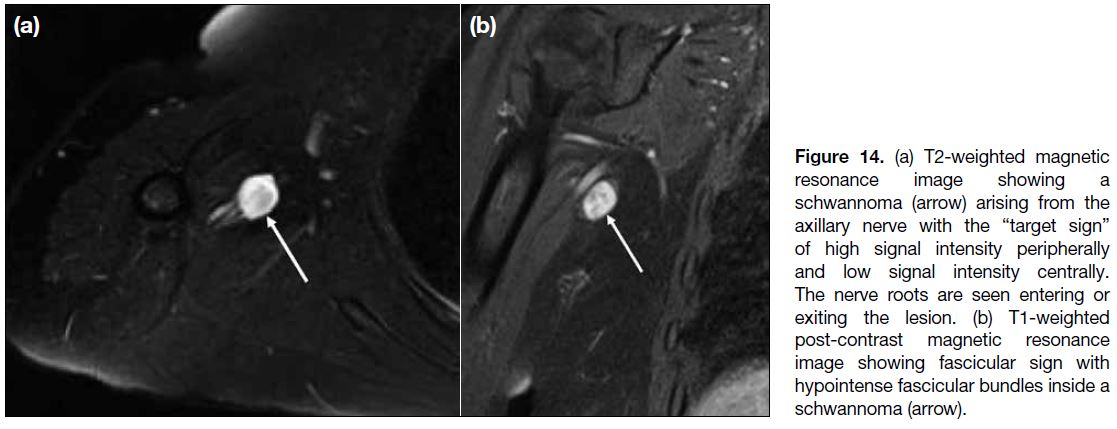
NERVES
Peripheral nerve sheath tumours are divided into two
benign entities, neurofibroma and schwannoma, and
a malignant form, malignant peripheral nerve sheath
tumour.[15] MRI plays an important role in the identification
and characterisation of these tumours. They commonly
appear as fusiform lesions and demonstrate low to
intermediate signal intensity on T1-weighted sequence
and high signal intensity on T2-weighted sequence.[15]
Some other suggestive imaging features include the
“entering or exiting nerve sign”, the “split-fat” sign,
the “target sign” (Figure 14a), the “fascicular sign”
(Figure 14b), and atrophy of the muscles supplied by the
involved nerve.[15]
Figure 14. (a) T2-weighted magnetic resonance image showing a schwannoma (arrow) arising from the axillary nerve with the “target sign” of high signal intensity peripherally and low signal intensity centrally. The nerve roots are seen entering or exiting the lesion. (b) T1-weighted post-contrast magnetic resonance image showing fascicular sign with hypointense fascicular bundles inside a schwannoma (arrow).
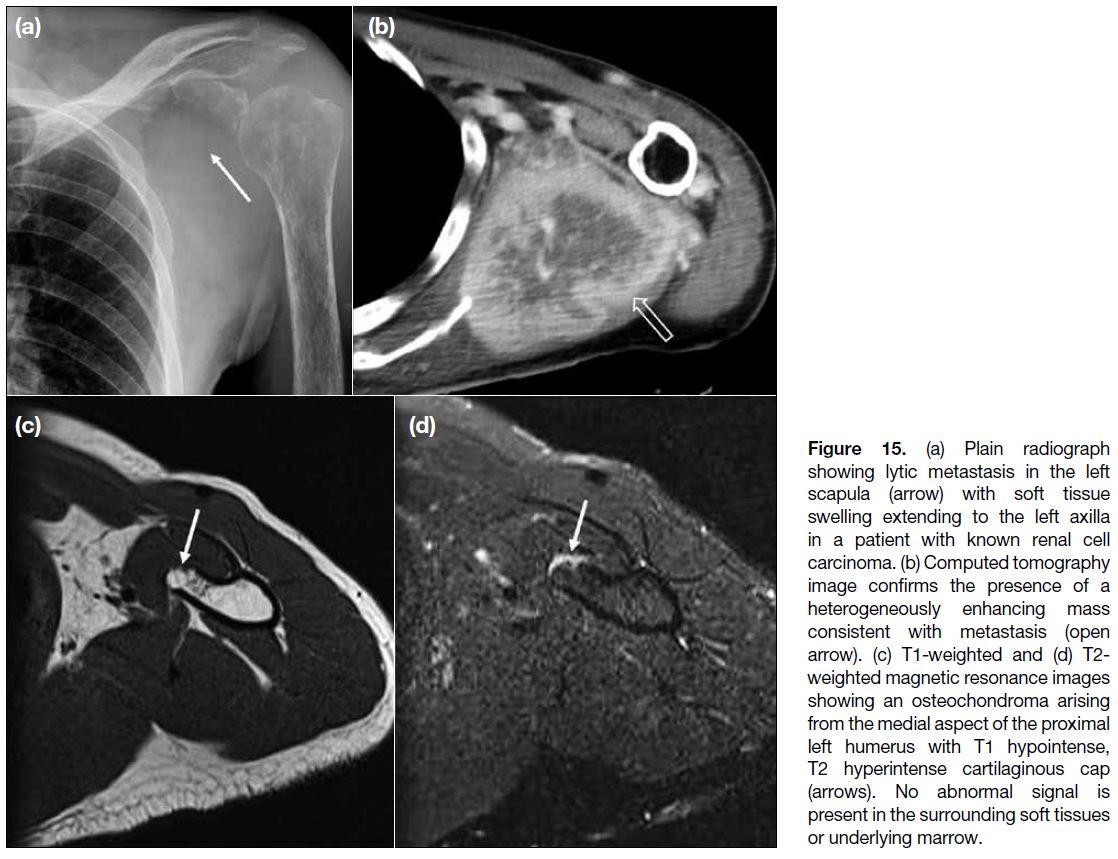
BONES
Tumours arising from bones such as the proximal
humerus, scapula or ribs, either primary or secondary,
aggressive or non-aggressive, can present as focal
swelling or mass in the axilla due to indentation or local
invasion. Examples include benign osteochondroma
(Figure 15a,b) and primary bone malignancy such as osteogenic sarcoma. Metastasis should be considered
especially in patients with a known history of primary
malignancy elsewhere (Figure 15c,d).
Figure 15. (a) Plain radiograph
showing lytic metastasis in the left scapula (arrow) with soft tissue swelling extending to the left axilla in a patient with known renal cell carcinoma. (b) Computed tomography image confirms the presence of a heterogeneously enhancing mass consistent with metastasis (open arrow). (c) T1-weighted and (d) T2-weighted magnetic resonance images showing an osteochondroma arising from the medial aspect of the proximal left humerus with T1 hypointense, T2 hyperintense cartilaginous cap (arrows). No abnormal signal is present in the surrounding soft tissues or underlying marrow.
CONCLUSION
Axillary lesions can be categorised as nodal or non-nodal
lesions. A relevant clinical history including any known systemic diseases, infection, previous breast
augmentation or recent vaccination may indicate
the underlying causes of axillary lymphadenopathy.
Familiarity with characteristic imaging findings such
as “snowstorm sign” for nodal silicone deposits, is also
useful. Lymph nodes with suspicious features warrant
follow-up or tissue diagnosis. For non-nodal lesions, one
should enquire if the patient has had any breast or axillary
intervention or active local infection. If such a history is
absent, the differential diagnoses can be narrowed down
according to the anatomical origin of the lesion.
REFERENCES
1. Oliff MC, Birdwell RL, Raza S, Giess CS. The breast imager’s approach to nonmammary masses at breast and axillary US: imaging technique, clues to origin, and management. Radiographics.
2016;36:7-18. Crossref
2. Gupta A, Metcalf C, Taylor D. Review of axillary lesions,
emphasising some distinctive imaging and pathology findings. J
Med Imaging Radiat Oncol. 2017;61:571-81. Crossref
3. Dialani V, James DF, Slanetz PJ. A practical approach to imaging the axilla. Insights Imaging. 2015;6:217-29. Crossref
4. Pinheiro DJ, Elias S, Nazário AC. Axillary lymph nodes in breast cancer patients: sonographic evaluation. Radiol Bras. 2014;47:240-4. Crossref
5. Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ,
Feldman MI, Madjar H, et al. ACR BI-RADS ultrasound. In:
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR
BI-RADS Atlas: Breast Imaging Reporting and Data System.
Reston: American College of Radiology; 2013.
6. Ikeda DM, Miyake KK. Breast Imaging: The Requisites. 3rd ed. Elsevier; 2017. p 422-5.
7. Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, et al. Unilateral axillary adenopathy in the setting of
COVID-19 vaccine. Clin Imaging. 2021;75:12-5. Crossref
8. Park YM, Park JS, Yoon HK, Yang WT. Imaging-pathologic
correlation of diseases in the axilla. AJR Am J Roentgenol.
2013;200:W130-42. Crossref
9. Wong T, Lo LW, Fung PY, Lai HY, She HL, Ng WK, et al.
Magnetic resonance imaging of breast augmentation: a pictorial
review. Insights Imaging. 2016;7:399-410. Crossref
10. Okubo M, Tada K, Niwa T, Nishioka K, Tsuji E, Ogawa T, et al.
A case of breast cancer in the axillary tail of Spence — enhanced
magnetic resonance imaging and positron emission tomography
for diagnostic differentiation and preoperative treatment decision.
World J Surg Oncol. 2013;11:217. Crossref
11. Giess CS, Raza S, Birdwell RL. Distinguishing breast skin lesions
from superficial breast parenchymal lesions: diagnostic criteria, imaging characteristics, and pitfalls. Radiographics. 2011;31:1959-72. Crossref
12. Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med. 2005;24:1397-402. Crossref
13. Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of
the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic
correlation. Radiographics. 2005;25:1371-95. Crossref
14. Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ,
Rubens DJ. Pseudoaneurysms and the role of minimally invasive
techniques in their management. Radiographics. 2005;25 Suppl
1:S173-89. Crossref
15. Chee DW, Peh WC, Shek TW. Pictorial essay: imaging of peripheral nerve sheath tumours. Can Assoc Radiol J. 2011;62:176-82. Crossref