Kidney and Inferior Vena Cava Abnormalities with Leg Thrombosis (KILT Syndrome) in a Young Healthy Male: a Case Report
CASE REPORT
Kidney and Inferior Vena Cava Abnormalities with Leg
Thrombosis (KILT Syndrome) in a Young Healthy Male:
a Case Report
LHQ Chin, M Cheung, GHT Ng, WWM Lam
Department of Radiology, Queen Mary Hospital, Hong Kong
Submitted: 11 Aug 2020; Accepted: 7 Oct 2020.
Contributors: All authors designed the study. LHQC acquired and analysed the data, and drafted the manuscript. MC, GHTN and WWML
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided written informed
consent for all treatments and procedures, and for publication of this case report.
INTRODUCTION
Kidney and inferior vena cava (IVC) abnormalities with
leg thrombosis (KILT syndrome) was first described in
the literature in 2002.[1] To date, several case reports have
described this rare phenomenon, often with incidental
detection of the triad of anomalies on cross-sectional
imaging. This radiological diagnosis is clinically
important because of the lifelong risk of recurrent venous
thrombosis, especially in young individuals, and the need
to screen for co-existing renal abnormalities. We present
the case of a 17-year-old male with newly diagnosed
KILT syndrome who presented with loin pain and lower
limb discomfort. Post-processing with volume and
cinematic rendering techniques were also incorporated
to illustrate the unique radiological findings.
CASE REPORT
A 17-year-old male presented to the hospital with a
2-week history of right loin pain and right lower limb
discomfort upon ambulation. Prior to admission, he had
developed increasing right loin and lower abdominal
pain. He had no significant medical history, relevant
risk factors or family history. Physical examination revealed right lower limb swelling with circumferential
difference.
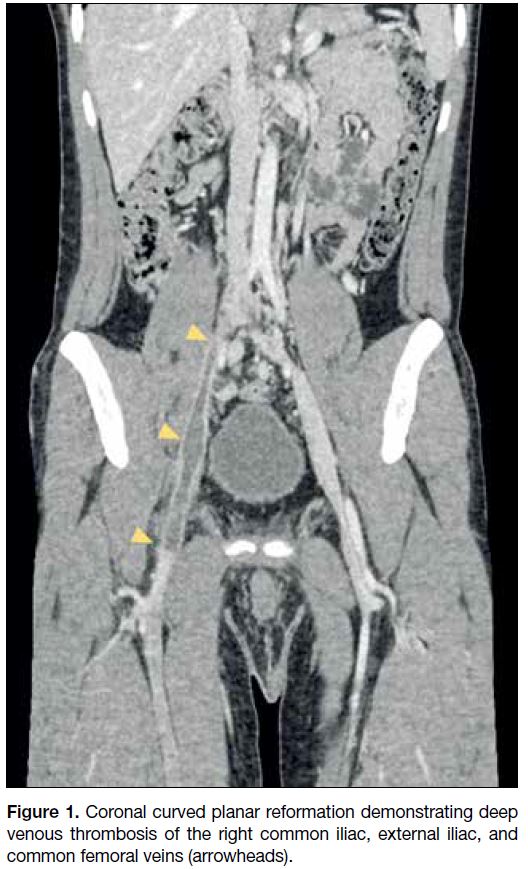
Computed tomography (CT) showed extensive deep
venous thrombosis (DVT) involving the right common
iliac, internal iliac, external iliac, and common femoral
veins (Figure 1). The infrahepatic segment of the IVC
appeared hypoplastic (0.8 cm calibre), whilst the
intrahepatic IVC was absent. Multiple dilated venous
collaterals were seen in the pelvis, retroperitoneum,
and along the lumbar epidural and paravertebral venous
plexuses. The azygos and hemiazygos veins were also
dilated (Figures 2 and 3). The right kidney was hypoplastic
(4.8 cm bipolar length) with compensatory hypertrophy
of the left kidney (13.0 cm bipolar length). The right
renal vein was patent but also hypoplastic in calibre.
The distended left renal vein was seen predominantly
draining into the distended hemiazygos vein (Figure 4).
Screening of the pulmonary arterial system showed no
evidence of pulmonary embolism.
Figure 1. Coronal curved planar reformation demonstrating deep
venous thrombosis of the right common iliac, external iliac, and
common femoral veins (arrowheads).
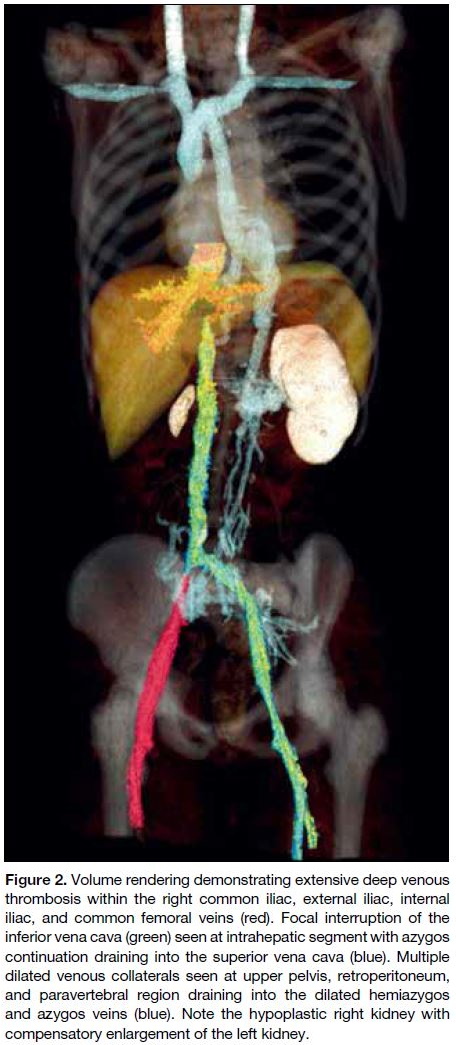
Figure 2. Volume rendering demonstrating extensive deep venous
thrombosis within the right common iliac, external iliac, internal
iliac, and common femoral veins (red). Focal interruption of the
inferior vena cava (green) seen at intrahepatic segment with azygos
continuation draining into the superior vena cava (blue). Multiple
dilated venous collaterals seen at upper pelvis, retroperitoneum,
and paravertebral region draining into the dilated hemiazygos
and azygos veins (blue). Note the hypoplastic right kidney with
compensatory enlargement of the left kidney.
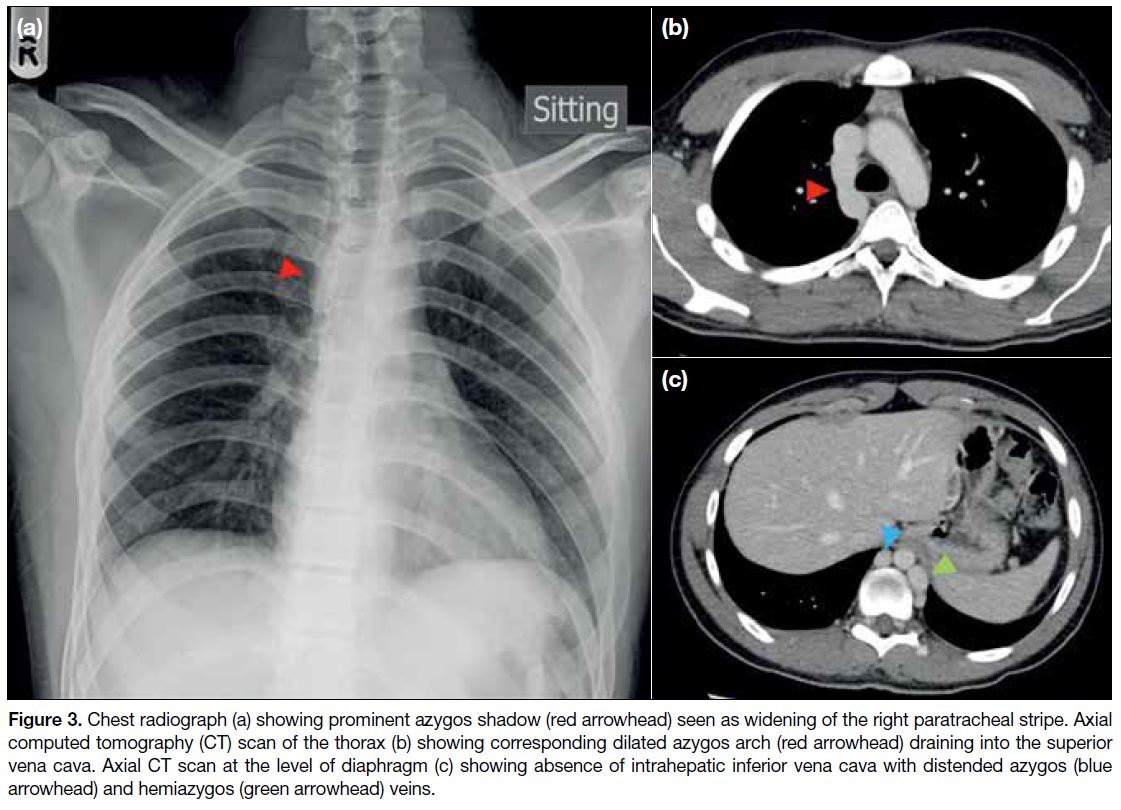
Figure 3. Chest radiograph (a) showing prominent azygos shadow (red arrowhead) seen as widening of the right paratracheal stripe. Axial
computed tomography (CT) scan of the thorax (b) showing corresponding dilated azygos arch (red arrowhead) draining into the superior
vena cava. Axial CT scan at the level of diaphragm (c) showing absence of intrahepatic inferior vena cava with distended azygos (blue
arrowhead) and hemiazygos (green arrowhead) veins.
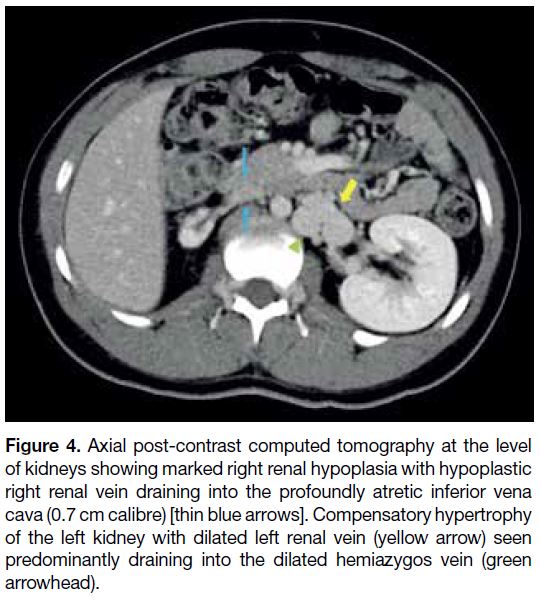
Figure 4. Axial post-contrast computed tomography at the level
of kidneys showing marked right renal hypoplasia with hypoplastic
right renal vein draining into the profoundly atretic inferior vena
cava (0.7 cm calibre) [thin blue arrows]. Compensatory hypertrophy
of the left kidney with dilated left renal vein (yellow arrow) seen
predominantly draining into the dilated hemiazygos vein (green
arrowhead).
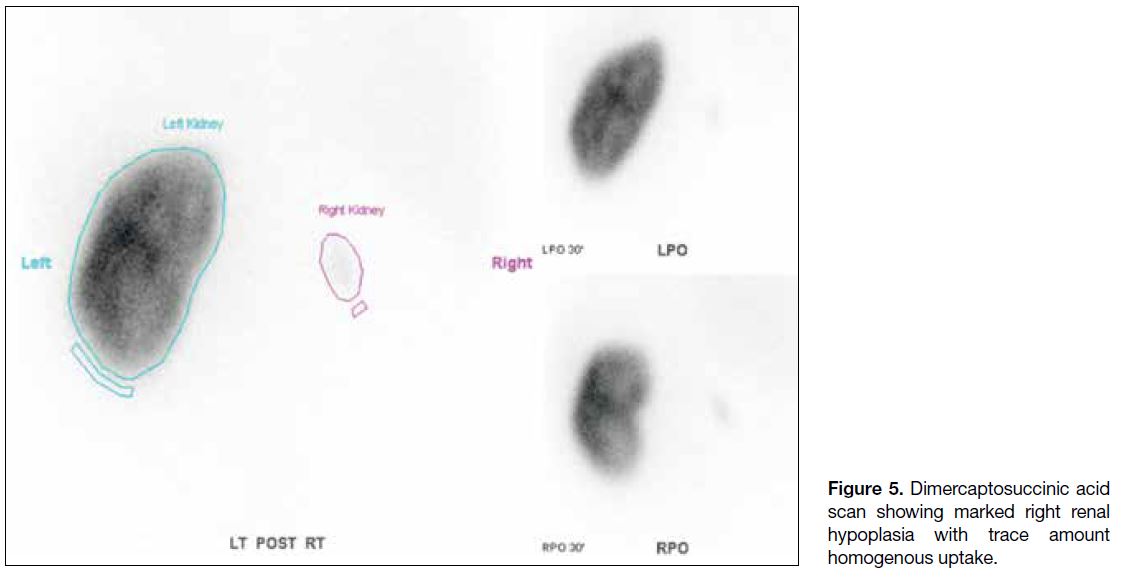
Radionuclide scan using technetium-99m combined with
dimercaptosuccinic acid showed small faint homogenous tracer uptake in the right kidney with differential renal
function of 99% and 1% for the left and right kidneys,
respectively (Figure 5).
Figure 5. Dimercaptosuccinic acid
scan showing marked right renal hypoplasia with trace amount homogenous uptake.
The patient’s renal function was normal with creatinine
of 95 μmol/L. Urinalysis showed no evidence of
microscopic haematuria or proteinuria. Extensive
thrombophilia workup was unremarkable.
Treatment was commenced with low molecular weight
heparin followed by oral anticoagulation (rivaroxaban
20 mg daily). Other supportive measures comprised
adequate hydration and analgesia. Upon discharge,
pain subsided with much improvement of right lower
limb swelling. Multidisciplinary follow-up care was
arranged, involving paediatricians, haematologists, renal
physicians, paediatric surgeons, physiotherapists and, occupational therapists. His joint long-term management
plan included lifelong anticoagulation medication,
regular outpatient monitoring of blood pressure, urine
protein and renal function, and advice to avoid contact
sports or strenuous exercises.
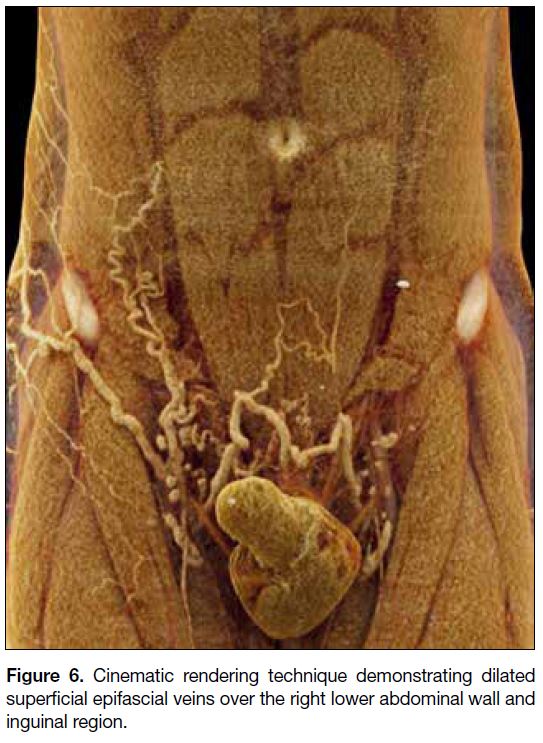
Interval follow-up CT scans revealed improvement of
the DVT with residual laminar thrombus seen in the right
common, external, and internal iliac veins. There was
interval development of dilated superficial abdominal
veins along the right lower abdominal wall and inguinal
region (Figure 6). The patient did not complain of
significant pain upon ambulation and had minimal
residual leg swelling.
Figure 6. Cinematic rendering technique demonstrating dilated superficial epifascial veins over the right lower abdominal wall and inguinal region.
DISCUSSION
In the paediatric population (aged 0-18 years), DVT
is rare, occurring in about 0.7 to 2.1 cases per 100 000
children compared with 100 to 150 per 100 000 adults.[2]
Given its low incidence in young patients, the index
of clinical suspicion may not be high despite a clinical
presentation of limb swelling and pain. As in our
case with spontaneous DVT, there is a quoted higher
incidence of underlying IVC anomalies (5%) compared
with the general population (0.5%).[3]
IVC anomalies are well-known independent risk factors
for DVT, occurring in up to 0.3% to 0.5% of the general population, and in 0.6% to 2% of those with underlying
cardiovascular defects.[4] The embryonic process of IVC
formation is complex, occurring between the fourth week
and eighth week of embryonic life. It is formed from three
sets of paired veins (supracardinal, posterior carinal, and
subcarinal veins).[5] In the literature, 15 to 60 different
IVC anomalies have been described. The more common
types include IVC duplication (most common, 2%-3%),
left-sided IVC, left retroaortic or circumaortic renal
vein and agenesis of the IVC.[4] IVC hypoplasia/agenesis
results in extensive collateral formation, most commonly
including the azygos, hemiazygos and lumbar veins,[6] [7] [8] as
similarly illustrated in our case. Relative to the literature,
our case would be referred to as “azygos continuation
of the IVC”.[9] These collaterals have inadequate venous
drainage despite structural enlargement with increased
venous pressure and stasis leading to increased risk for
recurrent DVT.[10]
Interestingly, clinical presentation of DVT can be
variable ranging from lower limb swelling and pain to
more atypical symptoms of loin or low back pain, as seen
in our case. However, these symptoms do not necessarily
suggest underlying KILT syndrome.[11]
Renal anomalies have been found to be associated
with IVC anomalies and leg DVT in a certain group of
patients. This triad was first described by Van Veen et al[1]
in 2002 and later named KILT syndrome.[9] A study by Sagban et al[12] found that right and left renal hypoplasia
were identified in 6% and 2.7% of IVC agenesis cases,
respectively. Likewise, as in our patient, the right kidney
is more commonly affected. This makes embryological
sense, as the venous return from the right metanephros
goes directly to the IVC whilst venous drainage of the
left metanephros is through the gonadal vein and lumbar
perforators.
Pulmonary embolism has been rarely reported in patients
with KILT syndrome or underlying IVC anomalies,
likely because the clot would need to be propelled via
the relatively small azygos and hemiazygos veins instead
of the IVC.[13]
Although ultrasound may diagnose lower extremity
DVT, it is not useful for detecting IVC anomalies. Chest
radiograph may show enlargement of the azygos shadow,
evidenced as widening of the right paratracheal stripe.
Contrast CT scan or magnetic resonance angiography
are preferred imaging modalities to diagnose KILT
syndrome. Dimercaptosuccinic acid renal scan will
provide information on differential renal function.
There is no clear consensus on the management of
KILT syndrome to date. Most case reports advocate
long-term anticoagulation due to the inherent lifelong
risk profile associated with IVC anomalies. Holistic
multidisciplinary care including analgesia, physical
rehabilitation, and active surveillance for young-onset
hypertension and renal function due to renal hypoplasia
are recommended.[11] Advice against physical exertion is
recommended since it may increase the risk for DVT
with underlying IVC anomalies.[13] As in our case, the
prospective long-term outcome and prognosis are yet to
be determined. More longitudinal follow-up studies are
required.
CONCLUSION
This case nicely illustrates a unique cause and risk factor
for DVT, occurring more commonly in paediatric and
young adult populations. The common co-existence of
kidney and IVC abnormalities also offers insight into the early in-utero and embryogenesis of KILT syndrome.
In a young patient who presents with idiopathic DVT
and no thrombophilia or apparent risk factors, further
imaging with CT or magnetic resonance angiography
is recommended to look for underlying pelvic/central
venous malformation and renal abnormalities.
REFERENCES
1. Van Veen J, Hampton KK, Makris M. Kilt syndrome? Br J
Haematol. 2002;118:1199-200. Crossref
2. Radulescu VC. Management of venous thrombosis in the pediatric
patient. Pediatric Health Med Ther. 2015;6:111-9. Crossref
3. Chee YL, Culligan DJ, Watson HG. Inferior vena cava
malformation as a risk factor for deep venous thrombosis in the
young. Br J Haematol. 2001;114:878-80. Crossref
4. Lauener S, Bütikofer A, Eigenheer S, Escher R. Thrombophlebitis
hiding under a KILT — case report on 40 years long-term follow-up
of neonatal renal vein thrombosis. BMC Pediatr. 2019;19:183. Crossref
5. Spentzouris G, Zandian A, Cesmebasi A, Kinsella CR,
Muhleman M, Mirzayan N, et al. The clinical anatomy of the
inferior vena cava: a review of common congenital anomalies and
considerations for clinicians. Clin Anat. 2014;27:1234-43. Crossref
6. Bami S, Vazquez Y, Chorny V, Goldfisher R, Amodio J. Deep
venous thrombosis of the leg, associated with agenesis of
the infrarenal inferior vena cava and hypoplastic left kidney
(KILT syndrome) in a 14-year-old child. Case Rep Pediatr.
2015;2015:864047. Crossref
7. Muntean C, Bucur G, Simu I, Tripon F, Marginean O. Deep venous
thrombosis associated with inferior vena cava abnormalities and
hypoplastic kidney in siblings. Acta Med Marisiensis. 2016;62:266-8. Crossref
8. Pomeranz CB, Cullen DL, Bellah RD. Deep venous thrombosis in a
child with inferior vena cava and renal anomalies: KILT syndrome.
Pediatr Radiol. 2018;48:1521-5. Crossref
9. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr.
Spectrum of congenital anomalies of the inferior vena cava: cross-sectional
imaging findings. Radiographics. 2000;20:639-52. Crossref
10. Ruggeri M, Tosetto A, Castaman G, Rodeghiero F. Congenital
absence of the inferior vena cava: a rare risk factor for idiopathic
deep-vein thrombosis. Lancet. 2001;357:441. Crossref
11. Fung JK, Yeung VH, Chu SK, Man CW. KILT (kidney and IVC
abnormalities with leg thrombosis) syndrome in a 41-years-old
man with loin pain and fever. Urol Case Rep. 2017;12:6-8. Crossref
12. Sagban TA, Scharf RE, Wagenhäuser MU, Oberhuber A,
Schelzig H, Grabitz K, et al. Elevated risk of thrombophilia in
agenesis of the vena cava as a factor for deep vein thrombosis.
Orphanet J Rare Dis. 2015;10:3. Crossref
13. Lambert M, Marboeuf P, Midulla M, Trillot N, Beregi JP,
Mounier-Vehier C, et al. Inferior vena cava agenesis and deep vein
thrombosis: 10 patients and review of the literature. Vasc Med.
2010;15:451-9. Crossref