Metformin Discontinuation for 48 Hours Reduces Intestinal Fluorodeoxyglucose Uptake in 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography
ORIGINAL ARTICLE
Metformin Discontinuation for 48 Hours Reduces Intestinal Fluorodeoxyglucose Uptake in 18F-fluorodeoxyglucose Positron
Emission Tomography/Computed Tomography
KK Ng, YH Hui, KS Chu, BT Kung, TK Au-Yong
Nuclear Medicine Unit and Clinical PET Centre, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr KK Ng, Nuclear Medicine Unit and Clinical PET Centre, Queen Elizabeth Hospital, Hong Kong. Email: nkk667@ha.org.hk
Submitted: 8 Jan 2020; Accepted: 19 Jun 2020.
Contributors: KKN and TKAY designed the study. KKN and YHH acquired the data. KKN analysed the data and drafted the manuscript. KKN,
YHH, KSC, and BTK critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Research Ethics Committee (Kowloon Central / Kowloon East) under Kowloon Central Cluster
of Hospital Authority, Hong Kong (Ref KC.KE-19-0048/ER-4).
Abstract
Objective
To evaluate the effect of 48-hour metformin discontinuation on bowel fluorodeoxyglucose (FDG) uptake
in a Chinese population undergoing 18F-fluorodeoxyglucose positron emission tomography/computed tomography
(FDG PET/CT) imaging.
Methods
All patients with known type 2 diabetes mellitus treated with metformin who had had a previous
FDG PET/CT examination performed in our centre, and who were scheduled for a second FDG PET/CT examination
within 1 year of the first one, were recruited. These subjects were advised to stop metformin for 48 hours prior to
the second examination. The intestinal uptakes were graded visually by a four-point scale and semiquantitatively
by maximum standardised uptake value (SUVmax) of small and large bowel segments. Any differences in intestinal
uptake, as well as other differences in examination day blood glucose levels between the two successive examinations
were compared.
Results
In total, 44 patients were included. Metformin discontinuation resulted in a significant reduction in small
and large bowel uptake by visual scoring. The SUVmax values were significantly lower in all bowel segments except
duodenum. Examination day blood glucose levels after 48-hour metformin discontinuation were <11 mmol/L, in all examinations.
Conclusion
Metformin discontinuation for 48 hours prior to scanning significantly reduced intestinal uptake and
should be considered as a method to improve PET/CT interpretation.
Key Words: Diabetes mellitus; Fluorodeoxyglucose F18; Metformin; Positron-emission tomography; Tomography,
X-ray computed
中文摘要
停用二甲雙胍48小時可降低FDG PET/CT中的腸道FDG攝取
吳官橋、許殷豪、朱競新、龔本霆、歐陽定勤
目的
在接受氟化去氧葡萄糖正電子及電腦雙融掃描(FDG PET/CT)成像的華籍人口中,評估停用二甲雙胍48小時對腸道FDG攝取的影響。
方法
納入所有接受二甲雙胍治療的已知2型糖尿病患者。他們曾於我們中心進行FDG PET/CT檢查,以及將於首次FDG PET/CT檢查後1年內進行第二次FDG PET/CT檢查,建議這些受試者在第二次檢查前48小時停用二甲雙胍。通過視覺評估將腸道FDG攝取程度分為四級,並通過小腸及大腸段的最大標準化攝取值 (SUVmax) 進行半定量。比較兩次連續檢查之間腸道FDG攝取的差異以及檢查日血糖水平的其他差異。
結果
共納入44例患者。視覺評估顯示停用二甲雙胍能顯著減少小腸及大腸FDG攝取。除十二指腸外,所有腸段的SUVmax值均顯著降低。在所有檢查中,停用二甲雙胍48小時後的檢查日血糖水平均<11 mmol/L。
結論
掃描前48小時停用二甲雙胍可顯著降低腸道攝取,可考慮作為改進PET/CT顯示的方法。
INTRODUCTION
Metformin is an oral antihyperglycaemic agent
commonly used in non-insulin dependent (type II)
diabetes mellitus. It is known to be associated with
typically intense, diffuse, and continuous uptake along
the bowel in 18F-fluorodeoxyglucose positron emission
tomography and computed tomography (FDG PET/CT).[1]
This can pose difficulty in FDG PET/CT interpretation
by obscuring bowel lesions or adjacent extra-intestinal
structures. In FDG PET/CT, a particular concern of
metformin discontinuation is the effect on blood glucose
levels, which, if >11 mmol/L,[2] may delay FDG PET/CT
scanning.
Previous studies on metformin discontinuation in non-Chinese populations had different study designs and
showed conflicting results in the effect of metformin
discontinuation on examination day blood glucose
levels.[3] [4] The purpose of this study was to evaluate the
effect of 48-hour metformin discontinuation on reducing
bowel FDG uptake in a Chinese population undergoing
FDG PET/CT, and the effect on blood glucose levels.
METHODS
Patient Recruitment
This was a prospective single-centre study with all
patients recruited from January 2019 to July 2019 who were referred to our PET/CT centre for ongoing
assessment of various neoplastic conditions. Inclusion
criteria were patients with type II diabetes being treated
with metformin as monotherapy or as part of polydrug
therapy, and who had undergone a previous FDG PET/CT
in our centre within the past year.
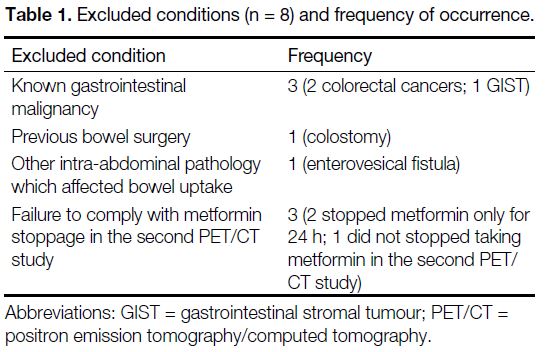
Exclusion criteria were patients with known
gastrointestinal malignancy, previous bowel resection,
or other intra-abdominal pathology that might affect
bowel uptake, e.g., an enterovesical fistula that might
cause the appearance of urinary excretion of FDG into
the bowel (Table 1).
Table 1. Excluded conditions (n = 8) and frequency of occurrence.
Patients were instructed to stop taking metformin for
48 hours prior to undergoing the second FDG PET/CT
study in our centre. Patients were interviewed on arrival
to PET/CT centre by nursing staff to confirm metformin
stoppage. Patients were also reminded to resume
metformin after PET/CT study was completed.
Patients’ blood glucose levels were checked and FDG
was only injected if they measured <11 mmol/L,
in accordance with local protocol and international
guidelines.[3] Other parameters, including body weight,
age, sex, metformin daily dosage, injected 18F-FDG
activity, and uptake time were recorded for each patient.
18F-fluorodeoxyglucose Positron Emission
Tomography and Computed Tomography
Acquisition
The two PET/CT studies were performed in each patient
using the same integrated PET/CT scanner (Discovery
710, General Electric, Milwaukee [WI], United States)
at Queen Elizabeth Hospital, Hong Kong. Patients were
instructed to fast for at least 6 hours before 18F-FDG
injection. Blood glucose levels were measured and the
FDG PET/CT was only performed if blood glucose level
was <11 mmol/L. Patients were injected with 370 MBq
(for normal-weight patients) or 555 MBq (for patients
with body weight >80 kg) according to department
protocol. Actual dose administered ranged from 347 to
609 MBq, as measured by dose calibrator. Acquisition
commenced 60 minutes after FDG administration. CT
scanning for anatomical localisation and attenuation
correction was performed with the following parameters:
120-kV tube voltage, 120-mA tube current, 0.5-s gantry
rotation time, and 0.984 pitch. The PET images were
acquired in three-dimensional mode, from skull to mid-thigh
with 2 minutes for each bed position. The PET raw
data were processed using ordered subset expectation
maximisation, point spread function modelling, and
time-of-flight (four iterations with 18 subsets and 5.5-mm
cut-off frequency). The data were reconstructed with
3.75-mm section thickness in a 256- × 256-mm matrix
and processed through a standard filter.
Image Analysis
Attenuation-corrected PET images, maximal intensity
projection images, CT images, and PET/CT fused
images were generated and displayed using a dedicated
workstation, AW VolumeShare 7 (AW 4.7 Ext. 8
Software, General Electric). The FDG uptake was graded
both visually and semiquantitatively over different bowel segments by an observer with 5 years of experience in
PET/CT.
Visual Analysis
A four-point score scale described by Gontier et al[1] was
used for visual assessment of the small and large bowel
FDG uptake: Grade 1 (lower than hepatic activity);
Grade 2 (similar to hepatic activity); Grade 3 (moderately
higher than hepatic activity); and Grade 4 (diffuse and
intense uptake).
Semiquantitative Analysis
Semiquantitative analysis of the FDG uptake over
different bowel segments was performed using maximum
standardised uptake value (SUVmax). The SUVmax was
measured using a region of interest of 1 cm in diameter
over the predefined anatomical locations, including:
horizontal portion of duodenum using the pancreatic
head as landmark; jejunum measured at mid-height of
descending colon; distal ileum adjacent to the ileocaecal
valve; caecum; hepatic flexure; splenic flexure; and
junction between descending colon and sigmoid colon.
Statistical Analysis
Statistical analysis was performed using SPSS (Windows
version 22.0; IBM Corp, Armonk [NY], United States).
A paired-sample t test was used for comparison of visual
analysis scores and SUVmax measurements between the
two successive PET/CT studies. A p value <0.05 was
considered significant.
RESULTS
Patient Characteristics
In total, 44 patients were recruited. Two patients who
stopped metformin only for 24 hours and one patient
who did not stop taking metformin in the second PET/CT study were excluded. A total of 41 patients (23 men,
18 women) with mean age of 67.2 ± 10.7 years were
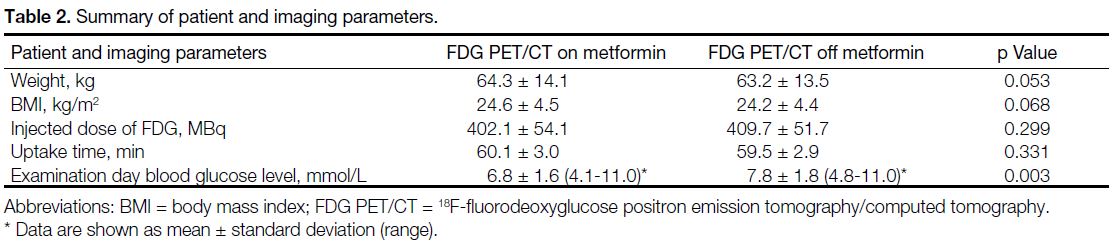
included in the final analysis. Patients’ parameters
including weight, body mass index, and metformin
daily dose between the two scans were unchanged. The
injected doses (402.1 MBq ± 54.1 vs. 409.7 MBq ± 51.7;
p = 0.299) and time between injection and imaging
between the two scans showed no statistically significant
difference (Table 2).
Effect of Metformin
Table 2. Summary of patient and imaging parameters.
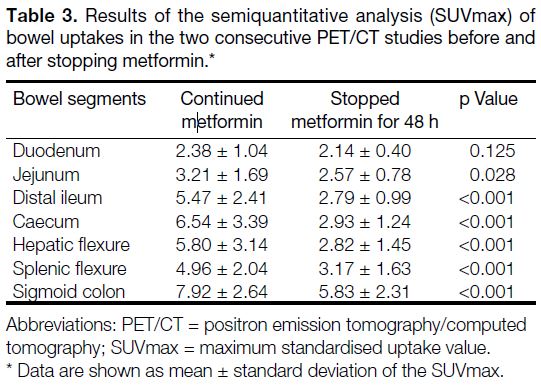
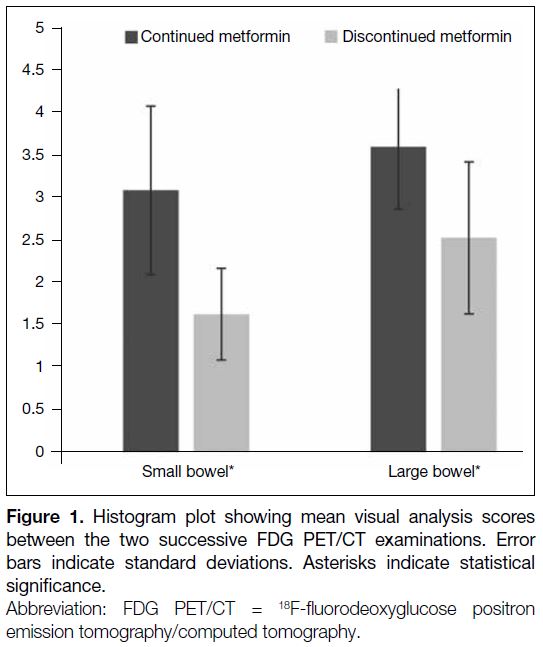
Small and large bowel FDG uptakes were significantly
reduced in the second FDG PET/CT study with
metformin stoppage for 48 hours, measured by visual scoring and SUVmax. Significant differences in visual
scoring of the bowel uptake were found in small
bowel (3.10 ± 1.00 vs. 1.63 ± 0.73) and large bowel
(3.61 ± 0.54 vs. 2.54 ± 0.90). For semiquantitative
analysis, there was a significantly lower SUVmax
over all the predefined bowel segments except at the
duodenum (Table 3, Figure 1).
Table 3. Results of the semiquantitative analysis (SUVmax) of bowel uptakes in the two consecutive PET/CT studies before and after stopping metformin.
Figure 1. Histogram plot showing mean visual analysis scores
between the two successive FDG PET/CT examinations. Error
bars indicate standard deviations. Asterisks indicate statistical
significance.
Effect of Metformin Discontinuation on
Examination Day Blood Glucose Level
Metformin daily dosage among the patients recruited
ranged from 500 to 2000 mg/d and were not changed
between the two PET/CT studies. A mean increase in
examination day blood glucose level of 1.07 mmol/L
was found when metformin was withheld for 48 hours.
All patients in this study had blood glucose levels
<11 mmol/L after discontinuation of metformin and did
not require injection of short-acting insulin.
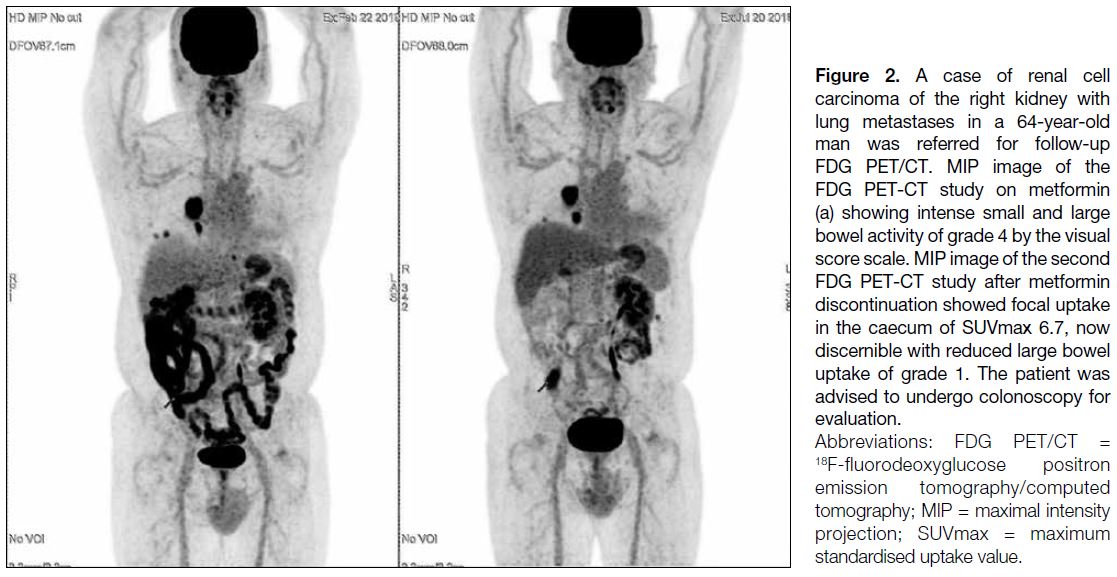
Illustrative Case with Focal Bowel Uptake
after Metformin Discontinuation
In a patient with history of renal cell carcinoma,
there was focal uptake in the caecum in the second
FDG PET/CT study performed after metformin discontinuation (Figure 2). This focal uptake was not discernible in the first FDG PET/CT with the presence
of metformin-related intense bowel activity. The large
bowel uptakes were scored Grade 4 and Grade 1,
respectively, in the two consecutive PET/CT studies
according to the visual analysis score.
Figure 2. A case of renal cell
carcinoma of the right kidney with lung metastases in a 64-year-old man was referred for follow-up FDG PET/CT. MIP image of the FDG PET-CT study on metformin (a) showing intense small and large bowel activity of grade 4 by the visual score scale. MIP image of the second FDG PET-CT study after metformin discontinuation showed focal uptake in the caecum of SUVmax 6.7, now discernible with reduced large bowel uptake of grade 1. The patient was advised to undergo colonoscopy for evaluation.
DISCUSSION
The exact mechanism of how metformin affects
intestinal glucose uptake and, hence, bowel FDG uptake,
is not entirely known. Ethnic differences in response to
metformin therapy have been reported.[5] [6] [7] Thus a local
population study to evaluate the effect of metformin on
bowel FDG uptake is warranted before implementing
changes in our imaging protocol. To the best of our knowledge, this is the first study in a Chinese population
that examined the effect of metformin discontinuation on
intestinal FDG uptake. Our study showed that metformin
discontinuation for 48 hours is effective in reducing
bowel FDG uptake in our local population with results
comparable to those of prior studies.[8] [9]
In FDG PET/CT study a practical consideration in
suspending metformin is the potential effect on blood
glucose levels on the day of the examination. According
to the European Association of Nuclear Medicine
procedure guidelines for tumour imaging, a FDG PET/CT
study should be delayed or rescheduled if the blood
glucose level is >11 mmol/L. This would create problems
in PET centre scheduling and, more importantly, a
delay in diagnosis. Contrary to previous study[6] which
asked patients to stop all antihyperglycaemic drugs, we
specifically asked patients to suspend only metformin,
whilst continuing other glucose-lowering medication(s).
Slightly higher examination day blood glucose levels
were observed with metformin discontinuation, but all
patients had blood glucose levels <11 mmol/L, thus
allowing timely performance of FDG PET/CT studies.
One merit of performing FDG PET/CT after metformin
discontinuation is that the reduced bowel uptake
facilitates potential bowel lesion detection, as illustrated
by a case in our study where an incidental focal colonic uptake in the caecum was only detected in
the second FDG PET/CT performed after metformin
discontinuation. The identification of focal colonic uptake
is of important clinical significance in FDG PET/CT,
as the reported pooled risk of malignant or pre-malignant
lesions appearing as focal colonic uptake
on FDG PET/CT was 68% in a meta-analysis[9] and the
authors stated that further investigation is warranted
whenever focal colonic uptake is detected. Similar
findings regarding focal colonic uptake and underlying
colonic polypoid lesion detection in FDG PET/CT have
also been reported in our centre, with a prevalence of
focal colonic uptake of 4.8%, comparable to previous
studies.[10] [11] It has been suggested that increased
bowel activity (SUVmax >5.9) encountered without
discontinuing metformin would obscure focal colonic
uptake detection and hinder appropriate management,
such as colonoscopy or virtual colonoscopy according to
an evidence-based review.[12] Further study is warranted
to evaluate the true incidence of focal colonic uptake
after metformin discontinuation.
There are limitations to our study. First, this was a single-centre study with a relatively limited number of patients
recruited. Second, it was a single-observer non-blinded
study, making it prone to observer bias. However, we
adopted the visual scale taking the liver uptake as an
internal reference, which is relatively consistent even in the presence of diffuse liver disease.[13] [14] [15] We also
predefined anatomical localisations of bowel segments to
reduce sampling bias and achieve higher reproducibility.
CONCLUSION
In summary, discontinuation of metformin for 48 hours
prior to FDG injection and scanning significantly reduced
bowel FDG uptake, which may facilitate bowel lesion
detection. It is an effective and feasible preparation
for FDG PET/CT studies and should be considered in
diabetic patients treated with metformin.
REFERENCES
1. Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 2008;35:95-9. Crossref
2. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K,
Eschner W, et al. FDG PET/CT: EANM procedure guidelines
for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging.
2015;42:328-54. Crossref
3. Hamidizadeh R, Eftekhari A, Wiley EA, Wilson D, Alden T,
Bénard F. Metformin discontinuation prior to FDG PET/CT: a
randomized controlled study to compare 24- and 48-hour bowel
activity. Radiology. 2018;289:418-25. Crossref
4. Lee SH, Jin S, Lee HS, Ryu JS, Lee JJ. Metformin discontinuation
less than 72 h is suboptimal for F-18 FDG PET/CT interpretation
of the bowel. Ann Nucl Med. 2016;30:629-36. Crossref
5. Mofo Mato EP, Guewo-Fokeng M, Essop MF, Owira PM. Genetic
polymorphisms of organic cation transporter 1 (OCT1) and
responses to metformin therapy in individuals with type 2 diabetes:
a systematic review. Medicine (Baltimore). 2018;97:e11349. Crossref
6. Maruthur NM, Gribble MO, Bennett WL, Bolen S, Wilson LM, Balakrishnan P, et al. The pharmacogenetics of type 2 diabetes: a systematic review. Diabetes Care. 2014;37:876-86. Crossref
7. Food and Drug Administration, US Government. GlumetzaTM,
500 mg (metformin hydrochloride extended release tablets) tablets,
film coated, extended release. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021748s002lbl.pdf. Accessed 24 Dec 2019.
8. Oh JR, Song HC, Chong A, Ha JM, Jeong SY, Min JJ, et al.
Impact of medication discontinuation on increased intestinal FDG
accumulation in diabetic patients treated with metformin. AJR Am
J Roentgenol. 2010;195:1404-10. Crossref
9. Treglia G, Taralli S, Salsano M, Muoio B, Sadeghi R, Giovanella L. Prevalence and malignancy risk of focal colorectal incidental uptake detected by 18F-FDG-PET or PET/CT: a meta-analysis. Radiol
Oncol. 2014;48:99-104. Crossref
10. Hui YH, Kung BT, Au Yong TK. Incidental focal colonic uptake
of 18F- fluorodeoxyglucose on positron emission tomography/computed tomography studies: its incidence and clinical
significance. Hong Kong J Radiol. 2020;23:275-80. Crossref
11. van Hoeij FB, Keijsers RG, Loffeld BC, Dun G, Stadhouders PH, Weusten BL. Incidental colonic focal FDG uptake on PET/CT:
can the maximum standardized uptake value (SUVmax) guide
us in the timing of colonoscopy? Eur J Nucl Med Mol Imaging.
2015;42:66-71. Crossref
12. Pencharz D, Nathan M, Wagner TL. Evidence-based management
of incidental focal uptake of fluorodeoxyglucose on PET/CT. Br J
Radiol. 2018;91:20170774. Crossref
13. Abele JT, Fung CI. Effect of hepatic steatosis on liver FDG
uptake measured in mean standard uptake values. Radiology.
2010;254:917-24. Crossref
14. Abikhzer G, Alabed YZ, Azoulay L, Assayag J, Rush C. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. AJR Am J Roentgenol. 2011;196:176-80. Crossref
15. Dostbil Z, Varoğlu E, Serdengeçti M, Kaya B, Onder H, Sari O. Evaluation of hepatic metabolic activity in non-alcoholic fatty livers
on 18FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2013;32:156-
61. Crossref