Imaging Findings of Progressing Rosai–Dorfman Disease of the Breast: a Case Report and Literature Review
CASE REPORT
Imaging Findings of Progressing Rosai–Dorfman Disease of the
Breast: a Case Report and Literature Review
WP Cheung, LW Lo, KM Wong, WS Mak, KM Kwok, EPY Fung
Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr WP Cheung, Department of Diagnostic & Interventional Radiology, Kwong Wah Hospital, Hong Kong. Email:
Submitted: 23 Sep 2019; Accepted: 18 Nov 2019.
Contributors: All authors designed the study, acquired the data, and analysed the data. WPC and LWL drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This case report was conducted in accordance with the Declaration of Helsinki. The patient provided consent for all tests and
procedures.
Acknowledgement: We thank Dr Kwan-Shun Ng for reviewing the pathological slides of biopsy and fine needle aspiration, preparing the
representative microphotos, preparing the legends and description of the microphotos, and providing comments and opinion on the pathological
findings of Rosai–Dorfman disease.
INTRODUCTION
Rosai–Dorfman disease (RDD) or sinus histiocytosis
with massive lymphadenopathy is a rare benign
proliferative disease of histiocytes with unclear aetiology.
Destombes first described the histological findings in
1965, and Rosai and Dorfman described the entity as
sinus histiocytosis with massive lymphadenopathy in
1969.[1] [2] Its classic manifestation is painless massive
cervical lymphadenopathy in young patients although
involvement of other nodal groups has also been
described. Extranodal involvement is common and
present in up to 43% of cases.[3] Exclusive extranodal
disease is less common, present in up to 23%.[3] Breast
parenchymal involvement is extremely rare with about
40 cases reported.[4] [5] RDD of the breast can mimic breast
cancer radiologically. To the best of our knowledge,
RDD of the breast with progressing radiological features
in addition to increase in number or size of masses
has not been described in the literature in the context
of suspected malignancy. We present such a case and
review the radiological and pathological features. It is
important to raise awareness of this rare but important
breast cancer mimicker.
CASE PRESENTATION
A 56-year-old lady with good past health and no family
history of breast cancer, presented with a 1-month
history of palpable right breast mass at the upper inner
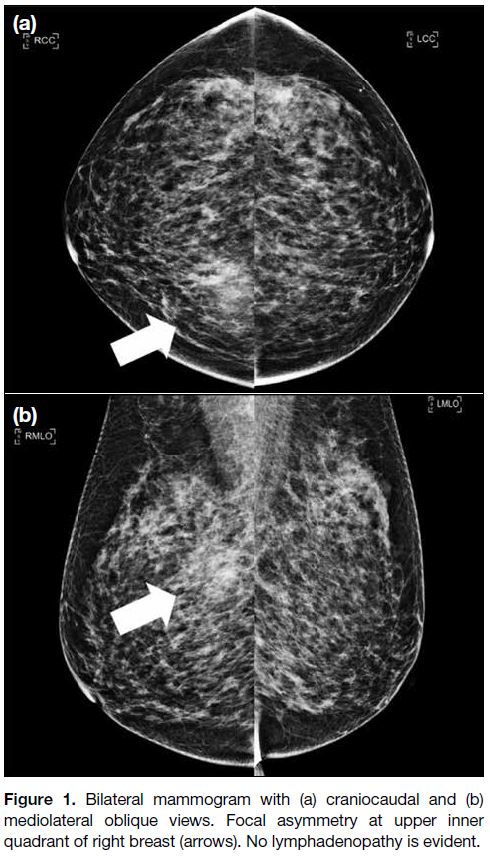
quadrant. Mammogram (MMG) with craniocaudal
and mediolateral oblique views demonstrated focal
asymmetry at the upper inner quadrant of the right breast
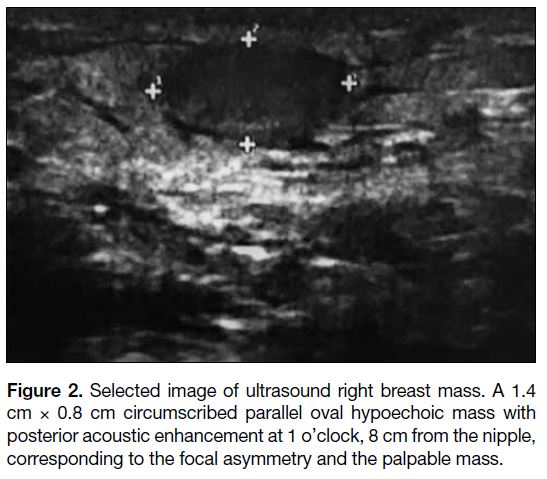
(Figure 1). Ultrasound (USG) revealed a 1.4 cm 0.8 cm
circumscribed parallel oval hypoechoic mass with
posterior acoustic enhancement at 1 o’clock, 8 cm from
the nipple, corresponding to the focal asymmetry seen
on MMG and the palpable mass (Figure 2). According
to the American College of Radiology Breast Imaging
Reporting and Data System (BI-RADS) 5th edition
the mass was classified as category 3: probably benign
(>0% but ≤2% likelihood of malignancy). The patient
was subsequently referred to breast surgery team for
management of palpable breast mass.
Figure 1. Bilateral mammogram with (a) craniocaudal and (b)
mediolateral oblique views. Focal asymmetry at upper inner
quadrant of right breast (arrows). No lymphadenopathy is evident.
Figure 2. Selected image of ultrasound right breast mass. A 1.4
cm × 0.8 cm circumscribed parallel oval hypoechoic mass with
posterior acoustic enhancement at 1 o’clock, 8 cm from the nipple,
corresponding to the focal asymmetry and the palpable mass.
Bedside USG 6 months later demonstrated interval
enlargement of the mass (2.2 cm 0.9 cm 2.6 cm) and
right axillary lymphadenopathy. Bedside USG-guided
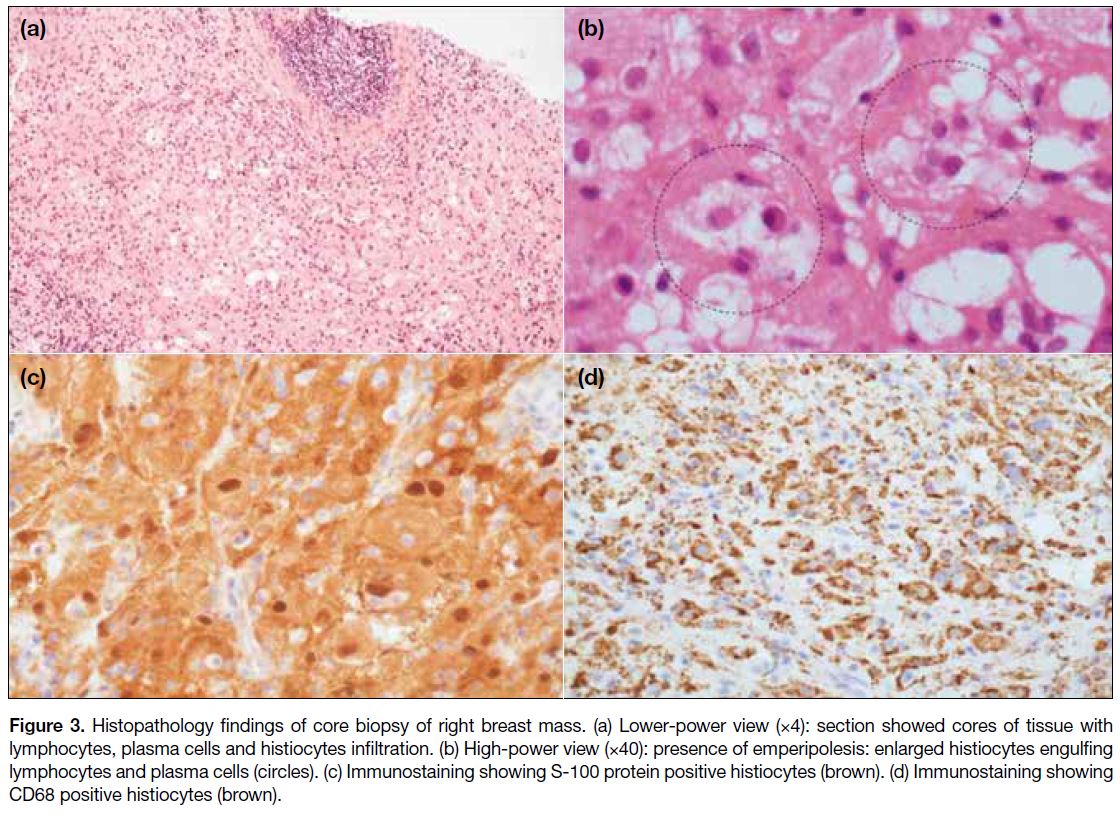
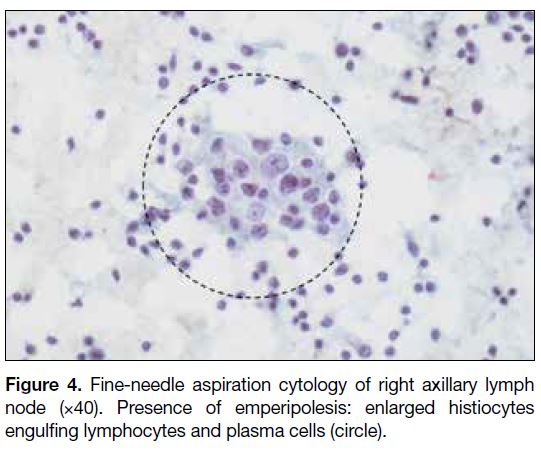
core biopsy of the mass and fine needle aspiration of right axillary lymphadenopathy was performed. Pathology
results showed features of RDD for both the right breast
mass and right axillary lymph node with no evidence
of malignancy (Figures 3 and 4). The patient opted for
conservative treatment with clinical and imaging follow-up.
Figure 3. Histopathology findings of core biopsy of right breast mass. (a) Lower-power view (×4): section showed cores of tissue with
lymphocytes, plasma cells and histiocytes infiltration. (b) High-power view (×40): presence of emperipolesis: enlarged histiocytes engulfing
lymphocytes and plasma cells (circles). (c) Immunostaining showing S-100 protein positive histiocytes (brown). (d) Immunostaining showing
CD68 positive histiocytes (brown).
Figure 4. Fine-needle aspiration cytology of right axillary lymph
node (×40). Presence of emperipolesis: enlarged histiocytes
engulfing lymphocytes and plasma cells (circle).
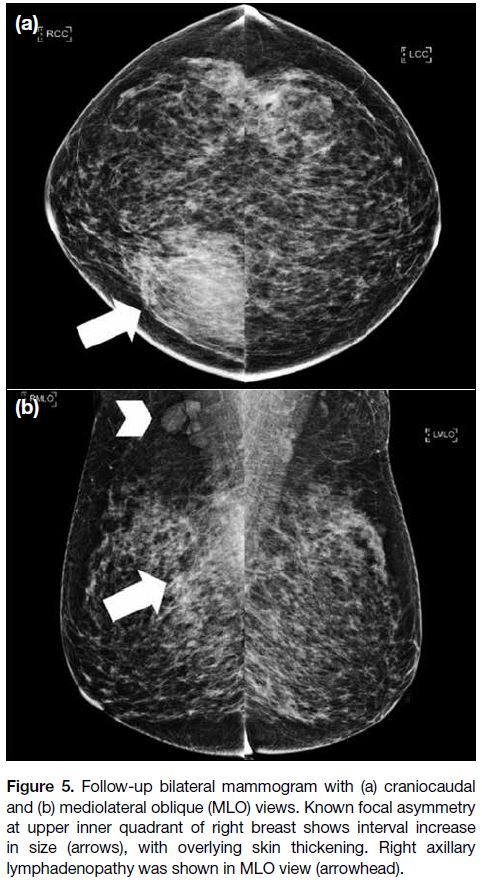
Follow-up MMG performed 2 years after the initial
presentation showed further interval increase in
size of the focal asymmetry and development of
associated overlying skin thickening. Right axillary
lymphadenopathy was also noted in the mediolateral
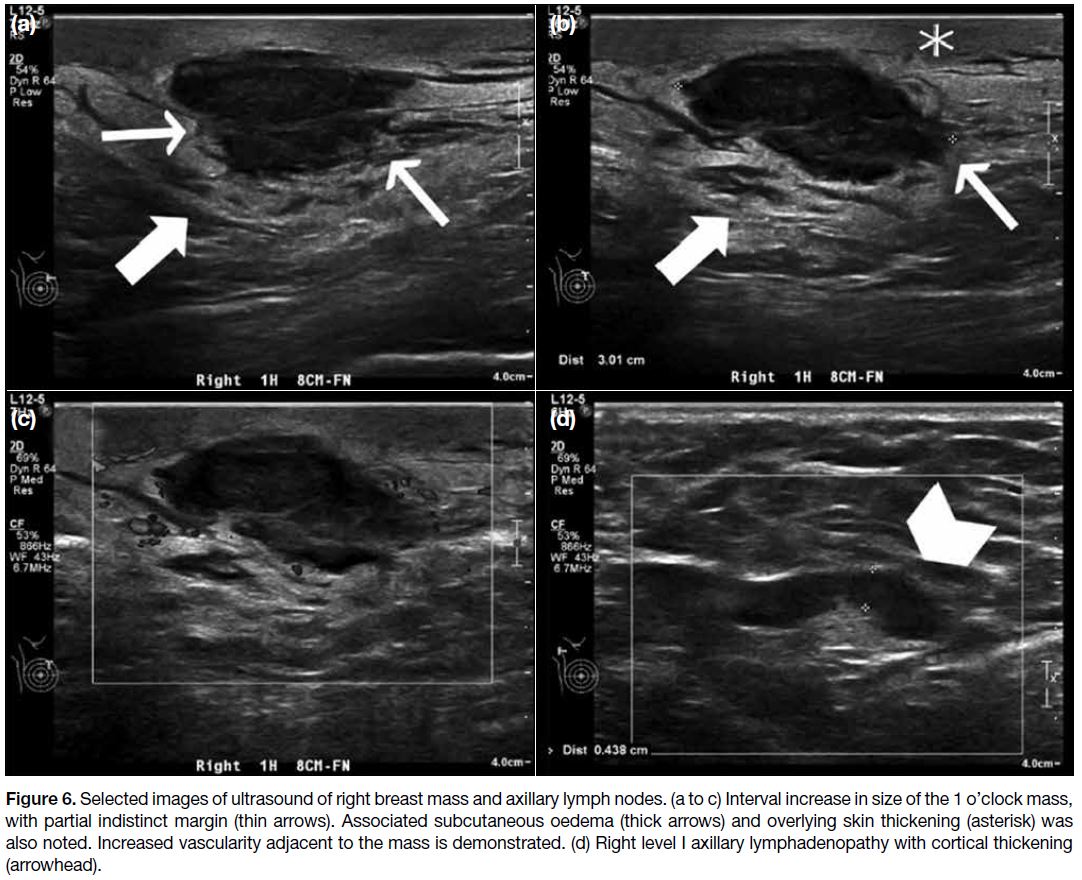
oblique view (Figure 5). USG showed interval increase
in size of the mass (2.6 cm 1.5 cm 3.0 cm) with
indistinct margin. Associated subcutaneous oedema and
overlying skin thickening was demonstrated. Doppler USG showed increased vascularity adjacent to the mass
(Figure 6a to c). Right level I axillary lymphadenopathy
with cortical thickening up to 0.4 cm was noted
(Figure 6d).
Figure 5. Follow-up bilateral mammogram with (a) craniocaudal
and (b) mediolateral oblique (MLO) views. Known focal asymmetry
at upper inner quadrant of right breast shows interval increase
in size (arrows), with overlying skin thickening. Right axillary
lymphadenopathy was shown in MLO view (arrowhead).
Figure 6. Selected images of ultrasound of right breast mass and axillary lymph nodes. (a to c) Interval increase in size of the 1 o’clock mass,
with partial indistinct margin (thin arrows). Associated subcutaneous oedema (thick arrows) and overlying skin thickening (asterisk) was
also noted. Increased vascularity adjacent to the mass is demonstrated. (d) Right level I axillary lymphadenopathy with cortical thickening
(arrowhead).
In view of the interval enlargement of the mass as well
as development of indistinct margin and skin changes
the mass was re-classified as BI-RADS assessment
category 4A: low suspicion for malignancy (>2% but
≤10% likelihood of malignancy). Tissue diagnosis was
suggested despite prior pathology showing RDD. Repeat
USG-guided core biopsy of the mass and fine needle
aspiration of right axillary lymphadenopathy were
performed. Pathology again showed features of RDD
with no evidence of malignancy.
The patient opted for conservative treatment. There were
no clinical signs or symptoms to suggest associated
or underlying conditions. No further investigations
including blood tests for inflammatory or autoimmune
markers were performed. She remains healthy 3 years
after the initial presentation.
DISCUSSION
The precise pathophysiology of RDD remains
unknown. It has been proposed to be related to inherited
conditions such as histiocytosis-lymphadenopathy plus
syndrome, neoplastic conditions such as lymphoma and
myelodysplastic syndrome, immune-related diseases
such as systemic lupus erythematosus, immunoglobulin
G4–related disease, and infection such as herpes simplex
virus 6.[5] [6]
RDD can manifest with a wide range of phenotypes.
The classic presentation is painless massive cervical
lymphadenopathy in children or young adult males.[4]
Other symptoms may include fever, night sweats, weight
loss, and organ-specific symptoms depending on the site
of involvement.[7] Extranodal disease is not uncommon,
present in up to 43% of cases; the most commonly
involved sites are skin and subcutaneous tissue, upper
respiratory tract, skeletal system and salivary glands.[3] [8]
Breast parenchymal involvement is extremely rare,
reported in only about 40 cases.[4] Cases with exclusive
breast involvement are mostly women aged >50 years
who present with single or multiple palpable lesions or an
abnormal screening MMG.[9] [10] Male breast involvement
has also been described.[7]
The imaging findings of RDD of the breast overlap
those of malignant lesions and occasionally mimic
other typically benign lesions. On MMG, RDD of
the breast can manifest as an irregular mass with
indistinct margins, a circumscribed mass or asymmetry.
Associated architectural distortion has also been
described.[3] [5] [11] On USG, it usually manifests as a
hypoechoic mass with indistinct or angulated margins.
Increased intralesional vascularity on Doppler USG is
also a reported feature.[5] Cases with sonographic features
mimicking cyst and fibroadenoma have been described
as well.[9] Mammographic and sonographic findings of
RDD of the breast are usually indistinguishable from
those of malignant lesions. They are usually classified as
BI-RADS category 4 or 5 and warrant tissue diagnosis.
Associated axillary lymphadenopathy is not uncommon
and present in up to 38% of cases.[5] In our patient, the
imaging features progressed at follow-up examination,
with interval increase in size of the mass, development
of indistinct margins, and skin changes, hence BI-RADS
assessment category was upgraded from 3 to 4A. To the
best of our knowledge, such progression has not been
described in the literature.
Laboratory findings of RDD are non-specific and include
elevated erythrocyte sedimentation rate, anaemia,
leucocytosis, and polyclonal hypergammaglobulinaemia.[3]
Definitive diagnosis relies on cytological evaluation of
fine needle aspiration, histopathological analysis of core
needle biopsy or surgical excision.[12] [13] Regardless of
the presence of nodal or extranodal disease, or the site
of involvement, RDD is characterised by aggregation
of large polygonal histiocytes. Pathological hallmark
features of this disease include emperipolesis (passage
of intact lymphocytes within intracytoplasmic vesicles
of histiocytes), S-100 protein and CD68 positivity, and
CD1a negativity.[12] [13]
It remains controversial whether patients with
proven RDD should be investigated for other sites of
involvement, including those with breast involvement.
Some authors suggest further evaluation with 18F-fluoro-2-deoxyglucosepositron emission tomography/computed
tomography, whole-body computed tomography, whole-body
magnetic resonance imaging, or selected imaging
based on symptoms of organ involvement, but no consensus
has been established.[5] [6] Others suggest that systemic
investigation is not necessary in patients with unifocal
RDD of the breast who are otherwise asymptomatic.[14]
The clinical course of RDD varies from spontaneous
regression, stable persistent disease, disease progression,
to fatality.[3] [8] [15] Risk factors associated with a poor
prognosis include involvement of larger number of
nodal groups, more extranodal system involvement,
involvement of the lower respiratory tract, liver or
kidneys, and immunological abnormalities.[3] [5] [6] The
clinical course of RDD of the breast remains uncertain
due to the limited number of reported cases.[11]
Treatment for RDD includes observation, corticosteroids,
immunomodulatory drugs, surgery, chemotherapy, and
radiotherapy.[6] However, there is no consensus on the
optimal management. In 2018, the Rare Histiocytoses
Steering Committee and Working Group of the
Histiocyte Society approved a management algorithm for
RDD. For asymptomatic extranodal disease, observation
was suggested since 20% to 50% of patients with nodal
or cutaneous disease will have spontaneous remission.
This is suitable for patients with uncomplicated
lymphadenopathy or asymptomatic cutaneous RDD
and potentially for those with asymptomatic disease
at other sites.[6] For symptomatic extranodal disease,
resection of single-site disease, which could be curative, systemic therapy for unresectable or multifocal disease
and resection/debulking of sites causing neurologic
or end-organ dysfunction are proposed.[6] However, the
proposed management algorithm does not specifically
address RDD of the breast: there is no proposed
follow-up schedule, and no comments about the need
for re-biopsy or subsequent management when there is
progression in size or radiological features of the breast
lesion in an otherwise asymptomatic patient, as in our
case. To the best of our knowledge, no reported cases
with a preoperative diagnosis of RDD of the breast was
subsequently upgraded after surgical excision. Whether
there is a need to re-biopsy and what is the appropriate
management when there is progression in size and
radiological features of a known breast RDD lesion
requires further study.[10] [14]
CONCLUSION
We report a case of RDD of the breast with progressing
radiological features. We describe the radiological
and pathological features and review this entity. It is
important to raise awareness of this rare but important
breast cancer mimicker.
REFERENCES
1. Destombes P. Adenitis with lipid excess, in children or young
adults, seen in the Antilles and in Mali (4 cases) [in French]. Bull
Soc Pathol Exot Filiales. 1965;58:1169-75.
2. Rosai J, Dorfman RF. Sinus histiocytosis with massive
lymphadenopathy. A newly recognized benign clinicopathological
entity. Arch Pathol. 1969;87:63-70.
3. Pham CB, Abruzzo LV, Cook E, Whitman GJ, Stephens TW.
Rosai-Dorfman disease of the breast. AJR Am J Roentgenol.
2005;185:971-2. Crossref
4. Goldbach AR, Hava S, Caroline D, Zhao X, Bains A, Pascarella S. Rosai-Dorfman disease of the breast: A potential marker of systemic
disease. Breast J. 2019:25;134-7. Crossref
5. Mar WA, Yu JH, Knuttinen MG, Horowitz JM, David O, Wilbur A,
et al. Rosai-Dorfman disease: manifestations outside of the head
and neck. AJR Am J Roentgenol. 2017;208:721-32. Crossref
6. Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile JF,
et al. Consensus recommendations for the diagnosis and clinical
management of Rosai-Dorfman-Destombes disease. Blood.
2018;131:2877-90. Crossref
7. Baladandapani P, Hu Y, Kapoor K, Merriam L, Fisher PR.
Rosai-Dorfman disease presenting as multiple breast masses in an
otherwise asymptomatic male patient. Clin Radiol. 2012;67:393-5. Crossref
8. Foucar E, Rosai J, Dorfman RF. Sinus histiocytosis with massive
lymphadenopathy (Rosai-Dorfman disease): review of the entity.
Semin Diagn Pathol. 1990;7:19-73.
9. Zhou Q, Ansari U, Keshav N, Davis F, Cundiff M. Extranodal
manifestation of Rosai-Dorfman disease in the breast tissue. Radiol Case Rep. 2016;11:125-8. Crossref
10. Delany EE, Larkin A, MacMaster S, Sakhdari A, DeBenedectis CM.
Rosai-Dorfman disease of the breast. Cureus. 2017;9:e1153. Crossref
11. Araujo JL, Cavalcanti BS, Soares MC, Sousa UW, Medeiros GP.
Rosai Dorfman’s disease in breast simulating breast cancer — A
care report. Caner Rep Rev. 2018;2:1-3. Crossref
12. Shi Y, Griffin AC, Zhang PJ, Palmer JN, Gupta P. Sinus
histiocytosis with massive lymphadenopathy (Rosai-Dorfman
disease): A case report and review of 49 cases with fine needle
aspiration cytology. Cytojournal. 2011;8:3. Crossref
13. Mantilla JG, Golberg-Stein S, Wang Y. Extranodal Rosai-Dorfman
disease clinicopathologic series of 10 patients with radiologic
correlation and review of the literature. Am J Clin Pathol.
2016;145:211-21. Crossref
14. Parkin CK, Keevil C, Howe M, Maxwell AJ. Rosai-Dorfman
disease of the breast. BJR Case Rep. 2015;1:20150010. Crossref
15. Bulyashki D, Brady Z, Arif S, Tsvetkov N, Radev RS. A fatal case
of Rosai-Dorfman disease. Clin Case Rep. 2017;5:1407-8. Crossref