Assessment of Commercially Available In-plane Bismuth Breast Shields for Clinical Use in Patients Undergoing Thoracic Computed Tomography
ORIGINAL ARTICLE
Assessment of Commercially Available In-plane Bismuth Breast Shields for Clinical Use in Patients Undergoing Thoracic Computed
Tomography
V Karami1, M Albosof2, M Najarian1, M Gholami3
1 Student Research Committee, Dezful University of Medical Sciences, Dezful, Iran
2 Department of Medical Engineering, Islamic Azad University, Dezful Branch, Iran
3 Department of Medical Physics, Lorestan University of Medical Sciences, Khorramabad, Iran
Correspondence: Dr M Gholami, Department of Medical Physics, Lorestan University of Medical Sciences, Khorramabad, Iran. Email: karami.ajums@yahoo.com
Submitted: 23 Feb 2019; Accepted: 27 May 2019.
Contributors: VK designed the study. VK and MA acquired the data. All authors analysed the data, drafted the manuscript and critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This study has been funded by the student research committee of Dezful University of Medical Sciences, Dezful, Iran (Grant
number: RC2.P1.1397). The funder had no role in study design, data collection, analysis or interpretation and manuscript preparation.
Ethics Approval: The study was approved by Dezful University of Medical Sciences Research Ethics Committee (Ref IR.DUMS.
REC.1397.053). The patients provided written informed consent.
Abstract
Objective
We aimed to assess qualitatively the effects of a bismuth breast shield by measuring image noise and
computed tomography (CT) number changes with 0-, 1-, 2-, and 3-cm shield-to-phantom distances. We also sought
to assess the dose reduction achieved by the shield and to evaluate its effect on image quality.
Methods
A cylindrical body phantom was scanned using an adult thoracic CT protocol with 0-, 1-, 2-, and 3-cm
foam spacers placed between the shield and the phantom, measuring the noise and CT numbers (in Hounsfield
units [HU]) of the image. We also used the shield with 3-cm spacer over the left breast in 180 female patients
referred for chest CT. Dose measurements were performed using thermoluminescent dosimeters. The image quality
was assessed following European guidelines.
Results
A 0-cm shield-to-phantom distance significantly increased noise and CT numbers of the image data. The 3-cm
shield-to-phantom distance effectively lowered shield-induced image noise; however, the HUs remained significantly
increased over all shield-to-phantom distances (p < 0.001). In the patient study, the average absorbed doses to the
shielded and non-shielded breasts were 13.6±3.1 mGy and 24.04±4.7 mGy, respectively; a 43.4% dose reduction.
Conclusion
Combining a bismuth shield with a 3-cm shield-to-breast foam spacer significantly reduced radiation
exposure without qualitative or quantitative deterioration of images in terms of image noise. However, increases in
the HUs of the images persisted.
Key Words: Bismuth; Radiation dosage; Radiation protection; Tomography, X-ray computed
中文摘要
商用縱切面鉍屏蔽對胸部電腦斷層掃描臨床使用的評估
Karami、M Albosof、M Najarian、M Gholami
目的
透過測量圖像噪聲以及水模至屏蔽距離為0、1、2和3厘米時的CT值變化來定性評估使用鉍屏蔽的效果,並評估鉍屏蔽對減少幅射劑量和圖像質量的影響。
方法
使用成人胸部CT協議掃描圓柱體模,於水模和屏蔽間放置0、1、2和3厘米的泡沫墊片,以測量圖像噪聲和其CT值。我們於180名轉診進行胸部 CT 的女性患者的左乳房上使用3厘米墊片的鉍屏蔽。使用熱釋光劑量計進行劑量測量,並根據歐洲指南評估圖像質量。
結果
水模至屏蔽距離為0厘米時,圖像噪聲和CT值顯著增加。水模至屏蔽距離為3厘米時能有效降低屏蔽引起的圖像噪聲;然而,CT值在所有水模至屏蔽距離上仍顯著增加(p < 0.001)。患者研究中,使用屏蔽和不使用屏蔽時乳房的平均幅射吸收劑量分別為 13.6 ± 3.1 mGy 和 24.04 ± 4.7 mGy,劑量減少 43.4%。
結論
結合鉍屏蔽與3厘米屏蔽至乳房泡沫墊片可顯著減少輻射暴露,且不會因圖像噪聲而定性或定量劣化圖像。然而,圖像的CT值持續增加。
INTRODUCTION
Computed tomography (CT) is an indispensable
diagnostic tool, providing cross-sectional and
3-dimensional images of anatomical structures with
exquisite anatomical detail.[1] During the last few years,
the number of CT examinations performed has steadily
increased.[2] [3] In 1980, three million CT scans were
performed in the United States.[4] This figure expanded to
57 million in 2000,[5] 62 million in 2007,[4] and 85 million in
2011.[6] A United States study in 2009 found that although
CT accounts for 11% of all radiological examinations,
it is responsible for 75.4% of the overall population
dose.[7] Concerns over exposure and overutilisation of
CT have resulted in several publications on the potential
risk of detrimental health effects.[8] [9] These studies have
linked CT with increasing the risk of radiation-induced
carcinogenesis, especially when radiosensitive tissues
are within the scan field.
Thoracic CT is a common examination that contributes
to radiation exposure to the breasts.[10] During thoracic
CT, the breasts receive approximately 17 to 22 mGy of
radiation.[11] [12] This is particularly due to their superficial
position that allows the breasts to be exposed to low-energy
scattered photons.[13] The breast tissue is highly
radiosensitive. Delivery of as little as 10 mGy to a young
woman is reported to increase the risk of radiationinduced
breast cancer by 13.6%.[3] Thoracic CT is intended to evaluate the lung parenchyma and mediastinum, and
often the breast is not under diagnostic evaluation.[3] [10]
Therefore, it is necessary that the radiation dose be kept
as low as reasonably achievable.[14]
The in-plane bismuth breast shield has shown to be
effective at reducing radiation exposure to the breasts
during thoracic CT.[3] [15] [16] Dose reductions of 26% to
61% have been reported in both phantom and clinical
studies using these shields.[5] [10] [16] [17] However, some
drawbacks, such as introduction of image noise, streak
and beam-hardening artefacts, and changes in CT
number (in Hounsfield units [HU]) of the images have
been concerns.[15] [18] [19] [20] It is suggested that placement of
a 1-cm thickness of foam or cotton between the shield
and the breast surface can reduce image noise.[21] Despite
variations in the dose reduction levels, there is agreement
in the literature on the potential radiation dose reduction
of bismuth shields, but their effect on image quality
remains controversial. Moreover, insufficient data exist
regarding the influence of the shield-to-breast distance
on image quality during thoracic CT.
The first aim of this study was to assess quantitatively
the effects on image noise and CT number changes in
image data following placement of 0-, 1-, 2-, and 3-cm
shield-to-phantom spacers in a homogenous body
phantom. The second aim was to assess dose reduction achieved by the shield and a qualitative assessment of
image quality based on image criteria adopted from the
European guidelines on quality criteria for thoracic CT.
METHODS
Phantom Study
A 32-cm cylindrical body water phantom (GE
Healthcare, Milwaukee [WI], US) was positioned at the isocentre of a 16-slice GE CT scanner (BrightSpeed,
GE Healthcare) and a scanogram was obtained to plan
the scanning range. The phantom was scanned using a
standard protocol routinely used for adult thoracic CT
(120 kVp, 100 effective mAs, 6-mm section thickness,
0.5 s/gantry rotation, 27-mm table increment per
rotation). A commercially available 0.06-mm lead
equivalent radioprotective bismuth breast shield (F&L
Medical Products Co., Vandergrift [PA], US) was
placed directly upon the anterior surface of the phantom
and a second scan was acquired. Subsequent scans were
acquired after placing a 1-, 2-, and 3-cm polyurethane
foam spacer between the shield and the anterior surface
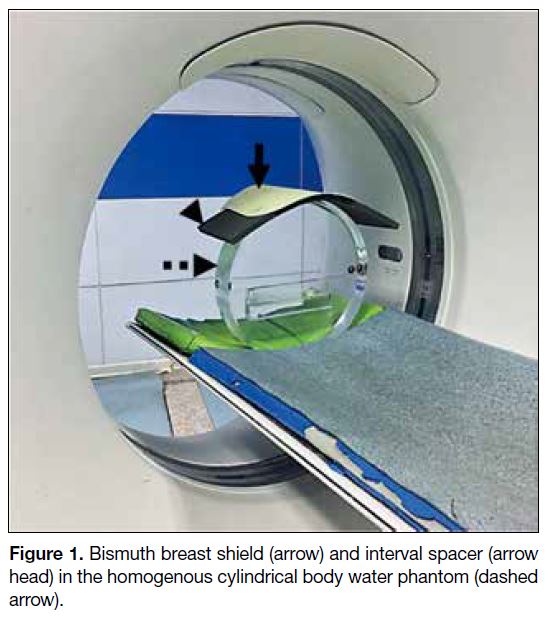
of the phantom (Figure 1). Identical scan parameters
were used for all acquisitions (Figure 2).
Figure 1. Bismuth breast shield (arrow) and interval spacer (arrow head) in the homogenous cylindrical body water phantom (dashed arrow).
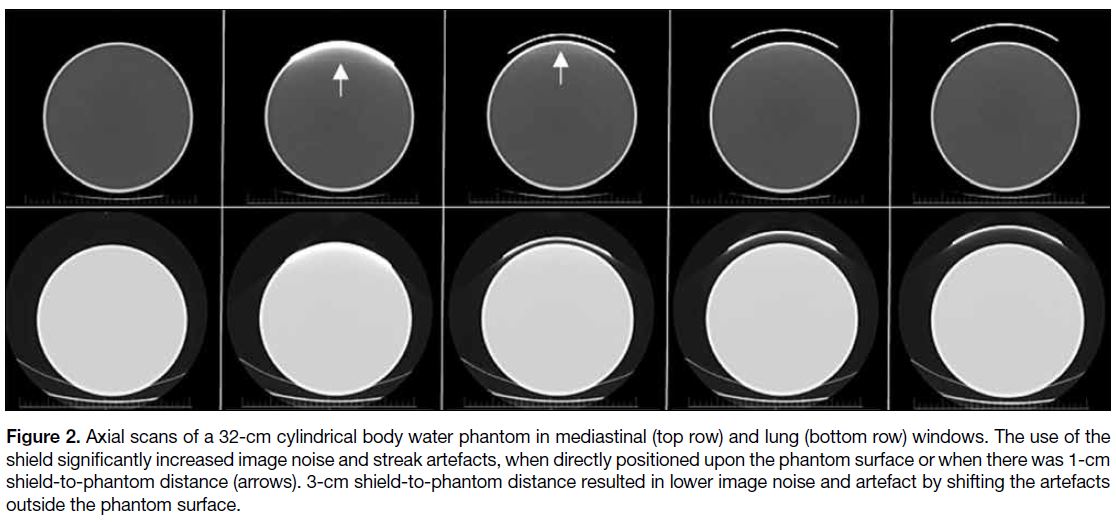
Figure 2. Axial scans of a 32-cm cylindrical body water phantom in mediastinal (top row) and lung (bottom row) windows. The use of the
shield significantly increased image noise and streak artefacts, when directly positioned upon the phantom surface or when there was 1-cm
shield-to-phantom distance (arrows). 3-cm shield-to-phantom distance resulted in lower image noise and artefact by shifting the artefacts
outside the phantom surface.
Quantitative Assessment of Image Noise in
the Phantom Study
To determine the influence of the shield and foam spacers
on image noise and HU variation, in each session, we
applied region of interest (ROI) methodology on three
consecutive axial slices. To obtain reliable results, three
sets of identical scans were obtained. Five circular ROIs
with areas of 1.5 cm2 were applied to each axial section
at the 12, 3, 6, and 9 o’clock positions plus one ROI in
the centre. The standard deviation of the density of ROIs
was considered as a quantitative analysis of image noise.
The mean HUs were recorded to determine variations caused by the shield.
Patient Study
Following approval from the university ethics committee
(approval number: IR.DUMS.REC.1397.053), 180
female patients (≥18 years) scheduled to undergo a
thoracic CT at our institution were recruited into the
study. Patients were considered eligible for inclusion
if they could follow the requirements of the study
for standard positioning (supine and arms above the
head) and had signed an informed consent form to
participate in the study as assigned by the ethics board.
All emergency studies and patients with unilateral or
bilateral mastectomies were excluded from the study.
Based upon our quantitative assessment of image noise
in the phantom study, we found that by using a 3-cm
shield-to-phantom distance, there was no significant
increase in noise or HU of the phantom images (except
the anterior part of the phantom) as compared to the case
where no shielding is applied. Therefore, we followed
this strategy in the patient study.
Thermoluminescent dosimeters (TLDs) [GR-200,
Hangzhou Freqcontrol Electronic Technology Ltd.,
China] were used for dose measurements. Before the
study, the TLDs were calibrated. Initially, all TLDs
were simultaneously irradiated with the same dose of
Cobalt-60 and then read out by a Harshaw 3500 TLD
reader (Harshaw, Solon [OH], US) and their element
correction coefficients were calculated. TLDs were
divided into 15 batches and exposed together with a 3-cm3
Radcal ionisation chamber (Radcal Corp., Monrovia,
[CA], US) at different doses in a diagnostic X-ray unit at
120 kV tube voltage. Following this, TLDs were read out
again and a calibration curve was generated to convert
the TLD charge in nanocoulombs to absorbed dose in
mGy. Before and after each use, TLDs were annealed
with a standard annealing regime recommended by the
manufacturer (245°C for 10 minutes).[22] [23] To prevent
the probable physical and chemical damage during the
dosimetry process, each TLD batch was placed in a thin
plastic bag. Throughout the study, three TLDs were used
as controls to measure background radiation. Patients
were positioned at the isocentre of the CT scanner in the
supine position. Images were acquired from the thoracic
inlet to the adrenal glands. To mark the approximate
adrenal region, the shadow of the kidneys was used
as a guide.[16] Following this, four fresh TLDs were
carefully placed on each breast (around the nipple since
it is relatively flat in supine position). A radioprotective
bismuth breast shield with a 3-cm shield-to-breast foam
spacer was placed over the left breast so that it covered
the entirety of the breast and the TLDs. The right breast remained non-shielded. The craniocaudal scan was
performed on the basis of the scanogram. The same
scanner and identical scan parameters were used in both
the phantom and clinical studies.
Qualitative Assessment of Image Quality in
the Patient Study
Three expert radiologists with a mean experience of
6 ± 2.3 years visually assessed the quality of patient
images based on image criteria adopted from the
European guidelines on quality criteria for thoracic
CT (Table 1).[24] Initially, the picture archiving and
communication system of the hospital was retrospectively
investigated to identify a reference thoracic CT (non-shielded)
in which each image quality criterion was
consistent with the European guidelines described in
Table 1. After obtaining one thoracic image as reference,
all 180 thoracic image datasets of the clinical study were
divided into left (shielded) and right (non-shielded)
sections in the 2-dimensional axial view as shown in
Figure 3. Each image quality criterion in each section
was compared by the identical criterion of the reference
image and graded as follows: (1) quality much lower
than reference image and diagnostically unacceptable;
(2) quality lower than reference image but diagnostically
acceptable; (3) quality equal to reference image, and (4)
quality better than reference image.
Table 1. European guidelines on quality criteria for thoracic computed tomography.
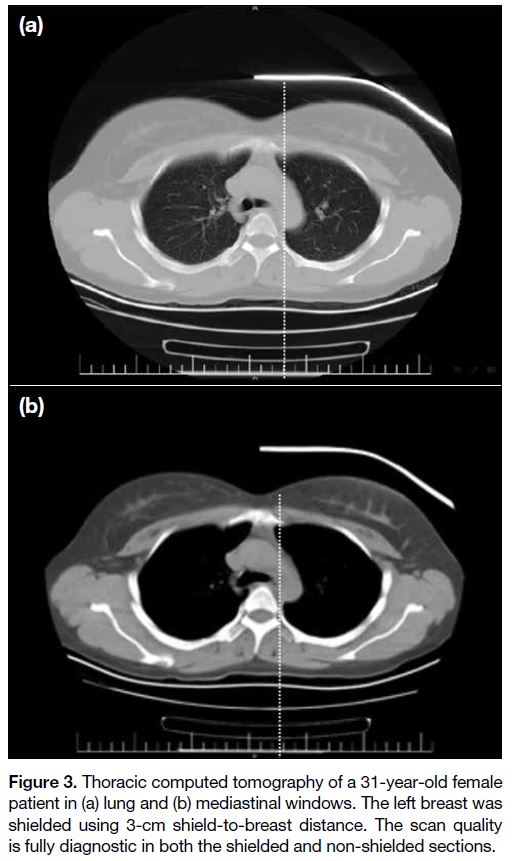
Figure 3. Thoracic computed tomography of a 31-year-old female
patient in (a) lung and (b) mediastinal windows. The left breast was
shielded using 3-cm shield-to-breast distance. The scan quality
is fully diagnostic in both the shielded and non-shielded sections.
Statistical Analysis
Data were entered into spreadsheet software (Excel,
Microsoft Inc., Redmond [WA], US) and statistical analysis was performed using a statistical package
SPSS (Windows version 20.0; IBM Corp, Armonk
[NY], US). The normality of the data was assessed
using the Kolmogorov–Smirnov test. To compare the
HU and image noise (standard deviation of HUs) of the
non-shielded phantom with 0-, 1-, 2-, and 3-cm shield-to-phantom
distances in each location (12, 3, 6, and 9 o’clock,
and centre), the Scheffe test (post hoc) was used with
alpha level of 0.05 and a confidence interval of 95%.
Student’s t test was used to compare the radiation dose
received by the breasts. A paired t test was used to
compare each image criterion of the shielded and non-shielded
sections of the thoracic images in terms of image
quality. A p value <0.05 was considered statistically
significant.
RESULTS
The influence of the shield and the different shield-to-phantom
distances on the quantitative image noise and
HU at the anterior, lateral, posterior, and central regions
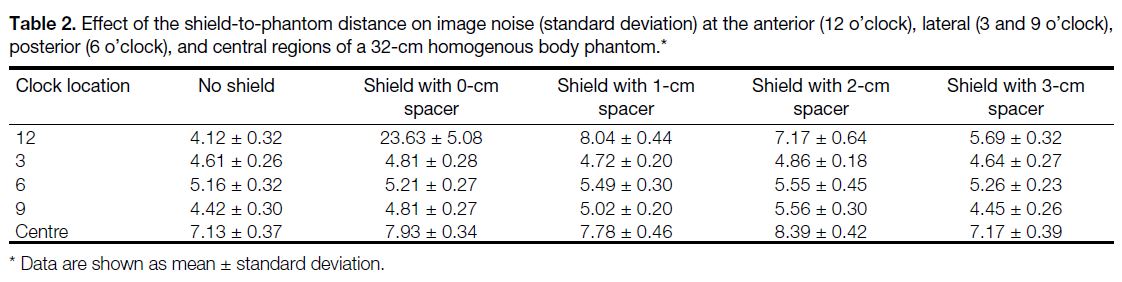
of the phantom images is summarised in Tables 2 and 3.
The increasing noise and HU of the shielded images was
progressively more pronounced at the anterior portion
of the phantom compared to the lateral, posterior, and
central regions. Increasing shield-to-phantom distance
resulted in lower image noise and HU variation. There
was no statistically significant difference between
shielded and non-shielded images in terms of image noise at all phantom regions when there was a 3-cm shield-to-phantom
distance (all p > 0.05). The shield increased HU
of the phantom images at all phantom regions (except
the posterior region) without a spacer and with all spacer
thicknesses. Streak artefacts were noted with no spacing
and with a 1-cm shield-to-phantom distance (Figure 2).
Table 2. Effect of the shield-to-phantom distance on image noise (standard deviation) at the anterior (12 o’clock), lateral (3 and 9 o’clock),
posterior (6 o’clock), and central regions of a 32-cm homogenous body phantom.
Table 3. Effect of the shield-to-phantom distance on Hounsfield Units at the anterior (12 o’clock), lateral (3 and 9 o’clock), posterior (6
o’clock), and central regions of a 32-cm homogeneous body phantom.
In the patient study, the patients’ age ranged from 18 to
74 years (mean, 41.5 ± 15.7). The mean absorbed dose
at the surface of shielded and non-shielded breasts was
13.6 ± 3.1 mGy and 24.04 ± 4.7 mGy, resulting in a
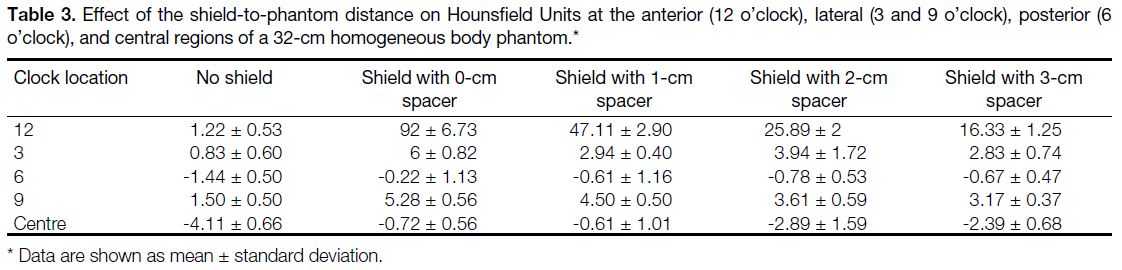
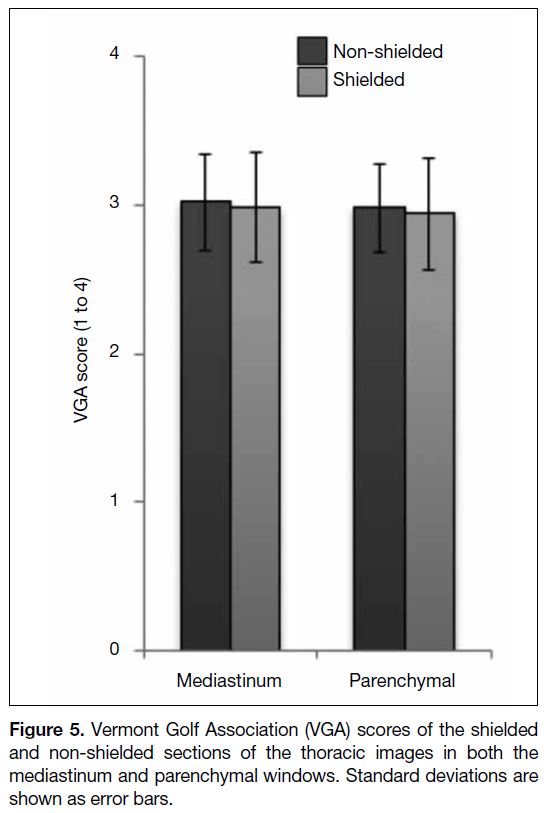
43.4% reduction in the breast dose (Figure 4). The mean
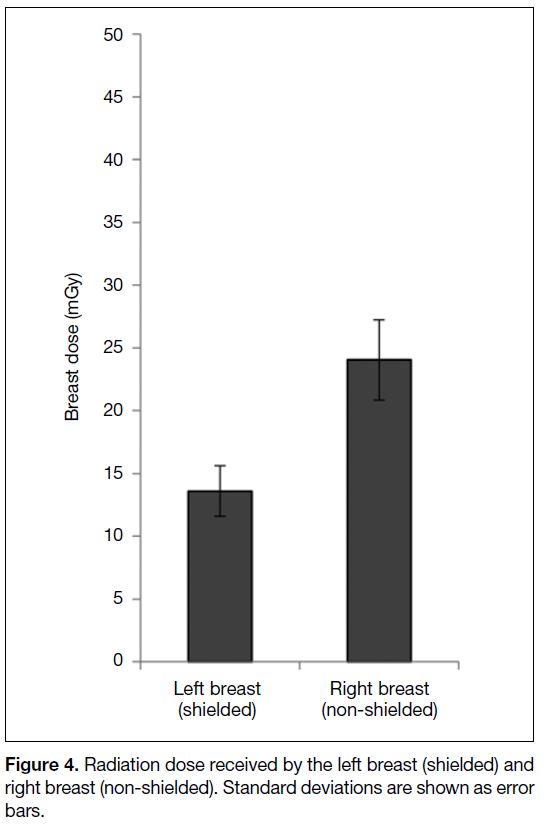
image quality scores in the shielded and non-shielded
sections of the thoracic images were 2.98 and 3.02 for
the mediastinal windows and 2.94 and 2.98 for the lung
windows, respectively (Figure 5) [p = 0.997]. All thoracic
images were interpreted as diagnostically acceptable.
Figure 4. Radiation dose received by the left breast (shielded) and
right breast (non-shielded). Standard deviations are shown as error
bars.
Figure 5. Vermont Golf Association (VGA) scores of the shielded
and non-shielded sections of the thoracic images in both the
mediastinum and parenchymal windows. Standard deviations are
shown as error bars.
DISCUSSION
The bismuth shield is reported to be an effective tool for
reducing radiation exposure of the breast during thoracic
CT.[16] However, some drawbacks such as increasing
noise level and HU of the images, especially in the
anterior thoracic region, have been reported.[15] [18] [19] [20]
In our quantitative assessment of image noise, we
demonstrated that a bismuth shield with no spacer
significantly increased noise and HU of the images at the anterior portion of the phantom. This result is
commensurate with the previous literature.[15] [25] [26] Our
result, however, is in contrast with that of Fricke et al,[12]
who reported no quantitative change in image noise
between shielded and non-shielded regions of the lung
for ≤18-year-old patients. This discordance may be due
to the fact that their study was performed on paediatric
patients that are typically scanned at low radiation
doses and therefore higher image noise was introduced,
whereas, in our study, the phantom was scanned with a
standard adult CT protocol that is associated with higher
radiation dose and lower image noise.[15] Consistent
with previous studies,[15] [27] [28] our study demonstrated that
increasing shield-to-phantom distance lowered noise in
the phantom images. When there was a 3-cm shield-to-phantom
distance, there was no statistically significant
difference between shielded and non-shielded images at all phantom regions in terms of image noise (all
p > 0.05); however, except in the posterior region,
the increase in HU was statistically significant in all
other phantom regions (p < 0.001). In a similar study,
Kalra et al[15] reported a significant HU increase at the
anterior and central portions of the images, with 0-, 1-,
2- and 6-cm shield-to-phantom distances. Kim et al[27]
reported a 19% to 40% image noise increase in the
anterior lung with a 1-cm shield-to-patient distance.
Similarly, Vollmar and Kalender[26] reported that bismuth
shielding with no spacer increased image noise up to
40%. The commercially available bismuth breast shields
have 1 cm of foam or cotton as a spacer between the
shield and the patient’s breast. We found that a 3-cm
shield-to-breast distance lowered image noise. However,
the increase in HU remains a concern.
According to our results, the mean radiation dose
delivered to the shielded breast was 13.6 mGy, which
represents a 43.4% reduction in the patients’ breast dose (24.04 mGy vs 13.6 mGy; p < 0.001). This result
is consistent with that of Yilmaz et al,[16] who reported a
40.5% reduction.
The assessment of image quality in the patient study
revealed no significant statistical difference between
images in the shielded and non-shielded sections of
the thoracic images in both the mediastinal and lung
windows (p = 0.362). We found no image criterion
reduced to the level of a diagnostically unacceptable
(score 1). All images were interpreted as diagnostically
acceptable. This result is commensurate with previous
clinical studies.[16] [29] 3-cm shield-to-breast distance
effectively shifts the artefacts arising from the shield
to the outside of the patient’s body. Previous studies
used phantoms and/or two separated groups of shielded
and non-shielded patients to assess image quality and
radiation dose reduction by the shield. We assumed that
our methodology may be more reliable than those studies
due to the fact that each measurement in patients had its
own control (the opposite breast).
There are also opinions siding against the use of bismuth
shielding during chest CT examinations. The American
Association of Physicists in Medicine has challenged
bismuth shielding, citing compromised image quality and
unpredictable and undesirable results when combining
with automatic exposure control (AEC).[30] Similarly, the
Society of Cardiovascular Computed Tomography has
avoided bismuth shielding due to its ability to influence
the accuracy of coronary calcification measurements
by increasing HU of the images.[31] Moreover, it has
been argued that the combination of the bismuth shield
with AEC would result in overestimating the patients’
attenuation and, consequently, offsetting bismuth shield
efficiency. Hence, if the shield is used in conjunction
with AEC, the shield should be placed after acquiring the
scanogram. In this situation, the desired image quality
may be slightly compromised but it does not influence
patient care. Moreover, it has been argued that there are
other dose reduction technologies such as organ-based
tube current modulation, global tube current reduction,
and iterative reconstruction techniques that do not have
the aforementioned drawbacks associated with bismuth
shielding but offer similar or even higher dose reduction
levels.[30]
According to this study, combining a bismuth shield with
a 3-cm shield-to-breast distance could effectively reduce
radiation exposure to the breast without quantitative
and/or qualitative deterioration of image quality in
terms of increasing noise and streak artefacts. However,
increasing the HU at the anterior, lateral and central
regions of the thorax remains a valid concern. According
to Kalra et al,[15] increasing the shield-to-phantom
distance up to 6 cm has also failed to eliminate this
drawback. The accuracy of HU is crucial for diagnosis
of some specific pathologies such as coronary artery
disease, which depends upon exact calcium density
measurement. Therefore, if the absolute accuracy of
the HU is necessary, the use of shielding should be
discouraged.
CONCLUSION
Combining a bismuth breast shield with a 3-cm spacer
significantly reduced radiation exposure to the breast
without qualitative or quantitative deterioration of the
image quality in terms of image noise and streak artefacts.
The bismuth shield was associated with increasing HU
of the images, not only in the anterior thorax but also
in the lateral and central regions. Therefore, when the
absolute accuracy of HU is crucial, the use of bismuth
breast shields should be discouraged.
REFERENCES
1. Kubo T, Ohno Y, Kauczor HU, Hatabu H. Radiation dose
reduction in chest CT — review of available options. Eur J Radiol.
2014;83:1953-61. Crossref
2. Lai NK, Liao YL, Chen TR, Tyan YS, Tsai HY. Real-time
estimation of dose reduction for pediatric CT using bismuth
shielding. Radiat Meas. 2011;46:2039-43. Crossref
3. Tappouni R, Mathers B. Scan quality and entrance skin dose
in thoracic CT: a comparison between bismuth breast shield
and posteriorly centered partial CT scans. ISRN Radiol.
2012;2013:457396. Crossref
4. Power SP, Moloney F, Twomey M, James K, O’Connor OJ,
Maher MM. Computed tomography and patient risk: facts,
perceptions and uncertainties. World J Radiol. 2016;8:902-15. Crossref
5. Coursey C, Frush DP, Yoshizumi T, Toncheva G, Nguyen G,
Greenberg SB. Pediatric chest MDCT using tube current
modulation: effect on radiation dose with breast shielding. AJR
Am J Roentgenol. 2008;190:54-61. Crossref
6. Miglioretti DL, Johnson E, Williams A, Greenlee RT,
Weinmann S, Solberg LI, et al. The use of computed tomography
in pediatrics and the associated radiation exposure and estimated
cancer risk. JAMA Pediatr. 2013;167:700-7. Crossref
7. Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al.
Exposure to low-dose ionizing radiation from medical imaging
procedures. N Eng J Med. 2009;361:849-57. Crossref
8. Lee CI, Forman HP. The hidden costs of CT bioeffects. J Am Coll
Radiol. 2008;5:78-9. Crossref
9. de González AB, Mahesh M, Kim KP, Bhargavan M, Lewis R,
Mettler F, et al. Projected cancer risks from computed tomographic
scans performed in the United States in 2007. Arch Intern Med.
2009;169:2071-7. Crossref
10. Parker MS, Kelleher NM, Hoots JA, Chung JK, Fatouros PP,
Benedict SH. Absorbed radiation dose of the female breast during
diagnostic multidetector chest CT and dose reduction with a
tungsten — antimony composite breast shield: preliminary results.
Clin Radiol. 2008;63:278-88. Crossref
11. Hopper KD, King SH, Lobell M, TenHave TR, Weaver JS. The
breast: in-plane x-ray protection during diagnostic thoracic CT
— shielding with bismuth radioprotective garments. Radiology.
1997;205:853-8. Crossref
12. Fricke BL, Donnelly LF, Frush DP, Yoshizumi T, Varchena V,
Poe SA, et al. In-plane bismuth breast shields for pediatric CT:
effects on radiation dose and image quality using experimental and
clinical data. AJR Am J Roentgenol. 2003;180:407-11. Crossref
13. Curtis JR. Computed tomography shielding methods: a literature
review. Radiol Technol. 2010;81:428-36.
14. World Health Organization. Communicating radiation risks in
paediatric imaging: information to support health care discussions
about benefit and risk. 2016. Available from: https://www.who.int/ionizing_radiation/pub_meet/radiation-risks-paediatr.... Accessed 7 Dec 2018.
15. Kalra MK, Dang P, Singh S, Saini S, Shepard JA. In-plane shielding
for CT: effect of off-centering, automatic exposure control and
shield-to-surface distance. Korean J Radiol. 2009;10:156-63. Crossref
16. Yilmaz MH, Albayram S, Yaşar D, Ozer H, Adaletli I, Selçuk D,
et al. Female breast radiation exposure during thorax multidetector
computed tomography and the effectiveness of bismuth breast
shield to reduce breast radiation dose. J Comput Assist Tomogr. 2007;31:138-42. Crossref
17. Geleijns J, Artells MS, Veldkamp W, Tortosa ML, Cantera AC.
Quantitative assessment of selective in-plane shielding of tissues
in computed tomography through evaluation of absorbed dose and
image quality. Eur Radiol. 2006;16:2334-40. Crossref
18. Servaes S, Zhu X. The effects of bismuth breast shields in
conjunction with automatic tube current modulation in CT imaging.
Pediatr Radiol. 2013;43:1287-94. Crossref
19. Einstein AJ, Elliston CD, Groves DW, Cheng B, Wolff SD,
Pearson GD, et al. Effect of bismuth breast shielding on radiation
dose and image quality in coronary CT angiography. J Nucl Cardiol.
2012;19:100-8. Crossref
20. McCollough CH, Wang J, Gould RG, Orton CG. Point/counterpoint. The use of bismuth breast shields for CT should be
discouraged. Med Phys. 2012;39:2321-4. Crossref
21. Hohl C, Wildberger JE, Süss C, Thomas C, Mühlenbruch G,
Schmidt T, et al. Radiation dose reduction to breast and thyroid
during MDCT: effectiveness of an in-plane bismuth shield. Acta
Radiol. 2006;47:562-7. Crossref
22. Hassanpour N, Panahi F, Naserpour F, Karami V, Asl JF,
Gholami M. A study on radiation dose received by patients
during extracorporeal shock wave lithotripsy. Arch Iran Med.
2018;21:585-8.
23. Behroozi H, Davoodi M, Aghasi S. Radiation dose to the thyroid
and gonads in patients undergoing cardiac CT angiography. Iran J
Radiol. 2015;12:e20619. Crossref
24. Jessen K, Panzer W, Shrimpton P, Bongartzm G, Geleijns J,
Golding S, et al. EUR 16262: European Guidelines on Quality
Criteria for Computed Tomography. Luxembourg: Office for
Official Publications of the European Communities. 2000.
25. Wang J, Duan X, Christner JA, Leng S, Yu L, McCollough CH.
Radiation dose reduction to the breast in thoracic CT: comparison
of bismuth shielding, organ-based tube current modulation, and use
of a globally decreased tube current. Med Phys. 2011;38:6084-92. Crossref
26. Vollmar SV, Kalender WA. Reduction of dose to the female
breast in thoracic CT: a comparison of standard-protocol, bismuth-shielded,
partial and tube-current-modulated CT examinations. Eur
Radiol. 2008;18:1674-82. Crossref
27. Kim YK, Sung YM, Choi JH, Kim EY, Kim HS. Reduced
radiation exposure of the female breast during low-dose chest
CT using organ-based tube current modulation and a bismuth
shield: comparison of image quality and radiation dose. AJR Am
J Roentgenol. 2013;200:537-44. Crossref
28. Lai CW, Cheung HY, Chan TP, Wong TH. Reducing the radiation
dose to the eye lens region during CT brain examination: the
potential beneficial effect of the combined use of bolus and a
bismuth shield. Radioprotection. 2015;50:195-201. Crossref
29. McLaughlin D, Mooney R. Dose reduction to radiosensitive tissues
in CT. Do commercially available shields meet the users’ needs?
Clin Radiol. 2004;59:446-50. Crossref
30. AAPM Position Statement on the Use of Bismuth Shielding for the
Purpose of Dose Reduction in CT scanning. Available from: https://www.aapm.org/publicgeneral/bismuthshielding.pdf. Accessed 7
Dec 2018.
31. Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M,
Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization
strategies in cardiovascular CT. J Cardiovasc Comput
Tomogr. 2011;5:198-224. Crossref