Magnetic Resonance Imaging Features of Cerebral Ring-Enhancing Lesions with Different Aetiologies: a Pictorial Essay
PICTORIAL ESSAY
Magnetic Resonance Imaging Features of Cerebral Ring-Enhancing Lesions with Different Aetiologies: a Pictorial Essay
KY Chan, JCW Siu
Department of Radiology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr KY Chan, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: Andrew_yuk@msn.com
Submitted: 15 Jan 2019; Accepted: 4 Mar 2019.
Contributors: KYC designed the study, acquired and analysed the data, and drafted the manuscript. All authors critically revised the manuscript
for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This pictorial essay received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by the New Territories West Cluster Research Ethics Committee (Ref NTWC/REC/19114). The
patients were treated in accordance with the tenets of the Declaration of Helsinki.
BACKGROUND
Cerebral ring-enhancing lesions are defined as an area of
hypodensity (in computed tomography) or hypointensity
(in magnetic resonance imaging [MRI]) of brain tissue
surrounded by a rim of enhancing tissue after contrast
injection. Presentation of diseases varies depending on
the site and extent of brain involvement and the aetiology.
It is always important to correlate clinical symptoms and
any previous imaging as the same imaging appearance
can suggest vastly different aetiologies with variation in
disease presentation. The aetiologies of cerebral ring-enhancing
lesions grossly include infectious, neoplastic,
post-treatment, demyelinating, and vascular causes. A
series of cases with definitive diagnosis based on clinical,
microbiological, or pathological evidence were retrieved
from our hospital database and their radiological
images reviewed. Multiple aetiologies of cerebral ring-enhancing
lesions will be discussed. Imaging features
will mainly focus on those shown on MRI.
INFECTIOUS CAUSES
Pyogenic Abscess
Pyogenic abscess is a potentially life-threatening disease
entity. Presentation includes septic symptoms, focal
neurological deficit depending on region of disease
involvement and symptoms of increased intracranial pressure. The disease usually presents rather quickly
with rapid deterioration over days. Predisposing factors
include those that facilitate haematological spread of
pathogens such as infective endocarditis and right to
left cardiac shunt with concomitant septic focus in other
regions.[1] The most common pathogen is Streptococcus
species and is identified in 35% to 50% of cerebral
pyogenic abscesses.[1] Regarding MRI features, the
central part of the lesion will be T1 hypointense and
T2 mildly hyperintense with no suppression on fluid-attenuated
inversion recovery (FLAIR) sequence.
Peripheral ring enhancement should be smooth and thin,
with the wall mostly being T1 and T2 hypointense.[2] One
of the features of pyogenic abscess is restricted diffusion
at the abscess cavity, with high diffusion-weighted
imaging (DWI) signal and lower apparent diffusion
coefficient signal. This is due to tightly packed cellular
content within the abscess cavity. Pyogenic abscess
often has marked surrounding vasogenic oedema with
mass effect that is significant compared with abscess size
(Figure 1). If it ruptures into the ventricles, ventriculitis
will ensue in which there will be intraventricular
debris at the dependent region with restricted diffusion
and enhancement along the ependymal surface of the
ventricle (Figure 2). The prognosis is often very poor in
such cases.
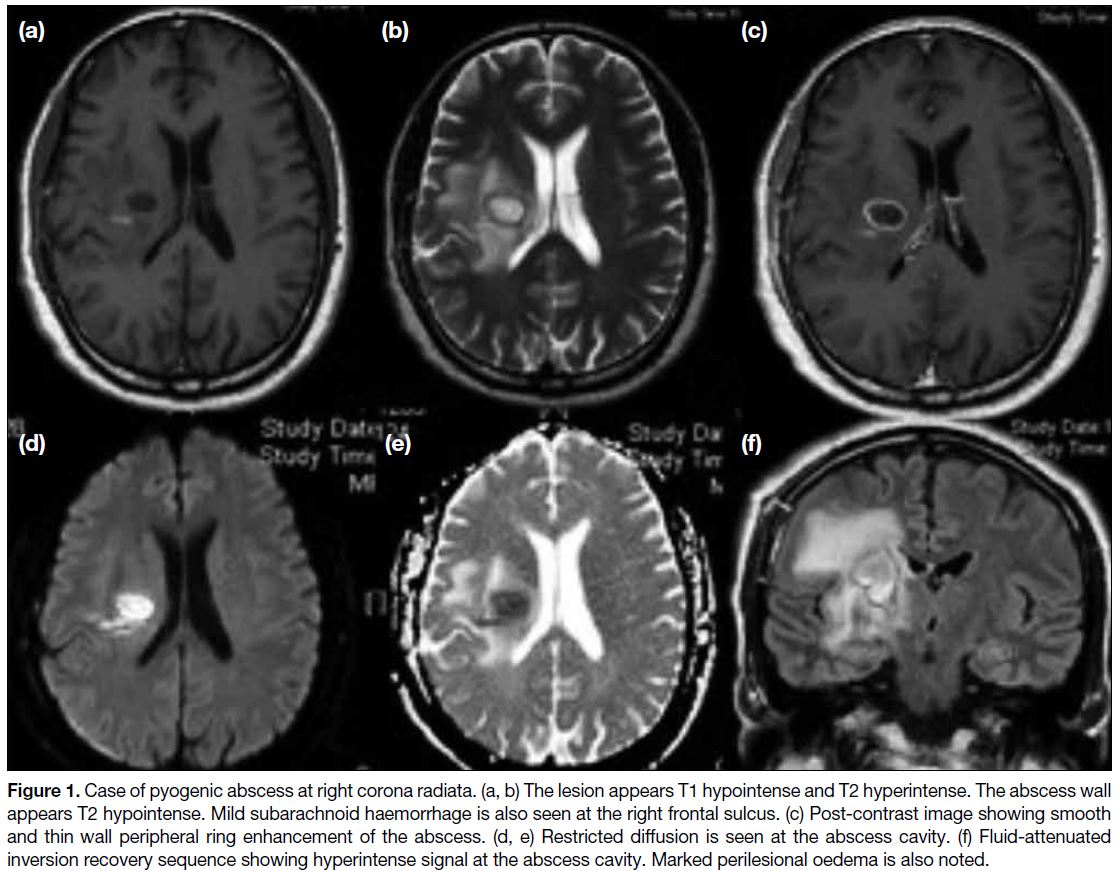
Figure 1. Case of pyogenic abscess at right corona radiata. (a, b) The lesion appears T1 hypointense and T2 hyperintense. The abscess wall
appears T2 hypointense. Mild subarachnoid haemorrhage is also seen at the right frontal sulcus. (c) Post-contrast image showing smooth
and thin wall peripheral ring enhancement of the abscess. (d, e) Restricted diffusion is seen at the abscess cavity. (f) Fluid-attenuated
inversion recovery sequence showing hyperintense signal at the abscess cavity. Marked perilesional oedema is also noted.
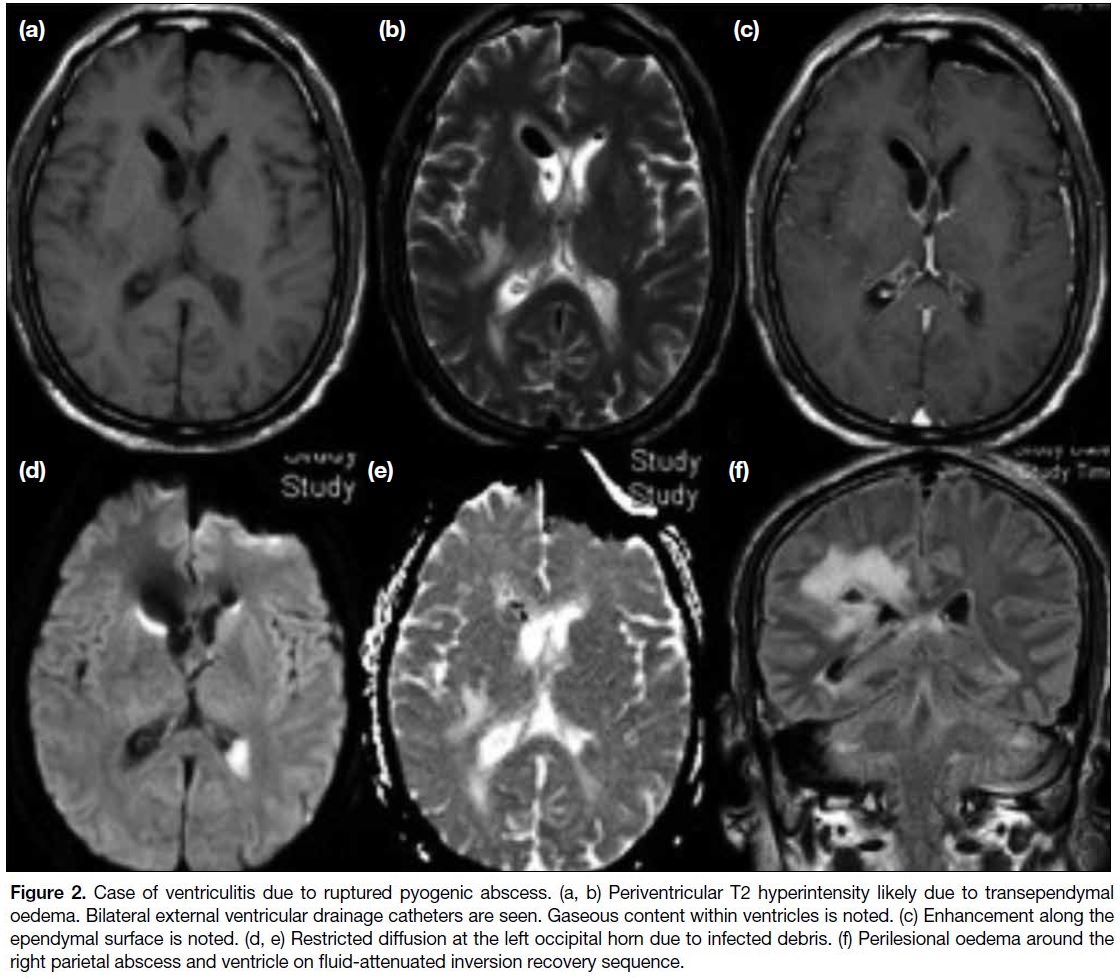
Figure 2. Case of ventriculitis due to ruptured pyogenic abscess. (a, b) Periventricular T2 hyperintensity likely due to transependymal
oedema. Bilateral external ventricular drainage catheters are seen. Gaseous content within ventricles is noted. (c) Enhancement along the
ependymal surface is noted. (d, e) Restricted diffusion at the left occipital horn due to infected debris. (f) Perilesional oedema around the
right parietal abscess and ventricle on fluid-attenuated inversion recovery sequence.
Tuberculoma
Tuberculoma is granulation tissue that occurs following
central nervous system (CNS) tuberculosis infection. It
differs to the much less common tuberculosis abscess that
is a true collection of pus.[3] It can occur with or without
tuberculous meningitis. When there is concomitant
tuberculous meningitis that predominantly affects the
basal cistern, the patient may present with cranial nerve
palsy. The lesion is usually T1 hypointense and T2
hypointense/isointense. However, T2 hyperintensity
may be noted when there is central liquified caseating
material.[4] Restricted diffusion is usually not a feature.
Enhancement pattern is usually ring-shaped but a mass-like
enhancing pattern may sometimes be seen. The
presence of concomitant basal cistern leptomeningeal
enhancement suggests meningitis with a high probability
of CNS tuberculosis as the underlying aetiology (Figure 3).
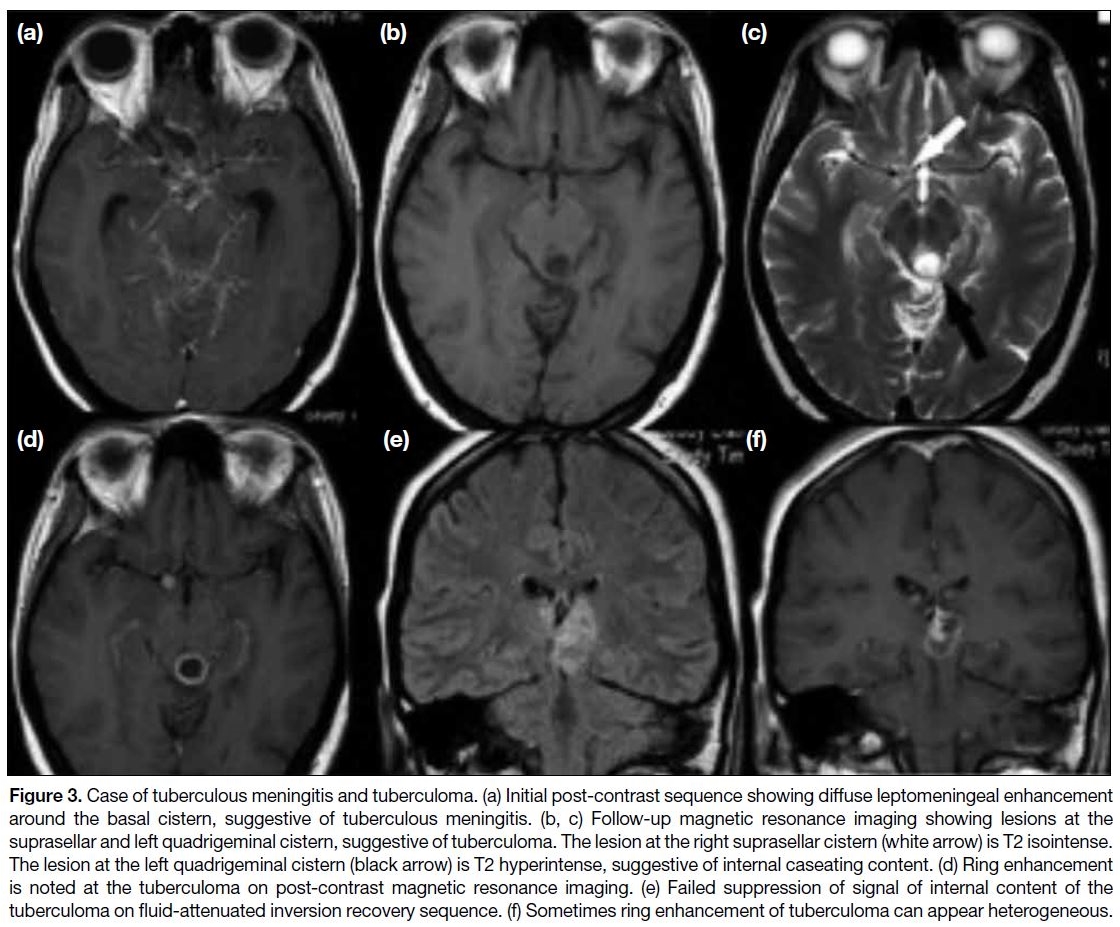
Figure 3. Case of tuberculous meningitis and tuberculoma. (a) Initial post-contrast sequence showing diffuse leptomeningeal enhancement
around the basal cistern, suggestive of tuberculous meningitis. (b, c) Follow-up magnetic resonance imaging showing lesions at the
suprasellar and left quadrigeminal cistern, suggestive of tuberculoma. The lesion at the right suprasellar cistern (white arrow) is T2 isointense.
The lesion at the left quadrigeminal cistern (black arrow) is T2 hyperintense, suggestive of internal caseating content. (d) Ring enhancement
is noted at the tuberculoma on post-contrast magnetic resonance imaging. (e) Failed suppression of signal of internal content of the
tuberculoma on fluid-attenuated inversion recovery sequence. (f) Sometimes ring enhancement of tuberculoma can appear heterogeneous.
Fungal Abscess
Fungal cerebral abscess is a rare cause of cerebral ring-enhancing lesion. It usually occurs in
immunocompromised individuals such as those
prescribed immunosuppressive therapies or having
undergone organ transplantation.[5] Morbidity and
mortality is high, and diagnosis should be made as soon
as possible. The estimated mortality is 85% to 100%.[6]
On MRI, the lesions are usually T1 hypointense and T2
hyperintense with no suppression on FLAIR sequence.
Fungal abscesses are more likely to be multiple with
involvement of the basal ganglia whereas pyogenic
abscesses are likely to be solitary and rarely involve
the basal ganglia.[6] One characteristic that has been
demonstrated in fungal abscess is restricted diffusion of
the abscess wall instead of the abscess cavity (Figure 4).
In pyogenic abscess, the cavity shows marked restricted
diffusion.[6] [7]
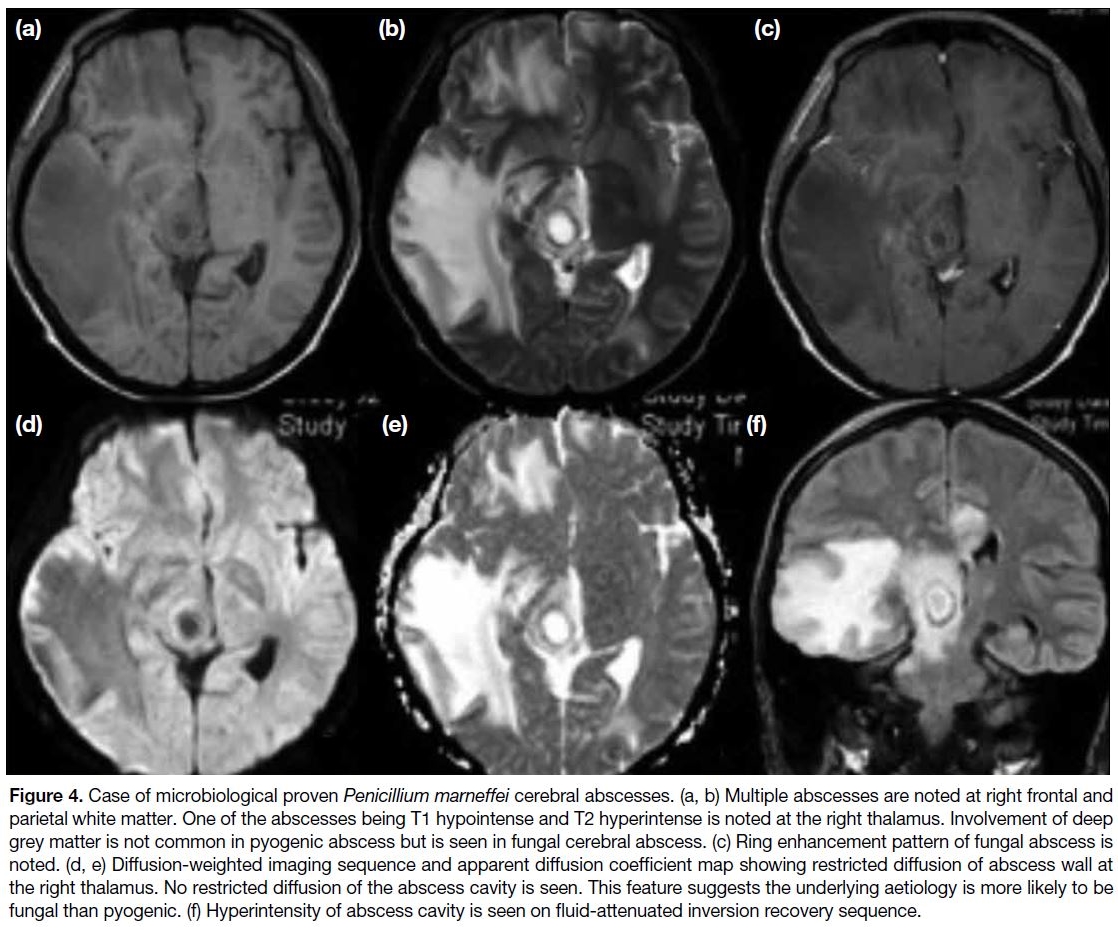
Figure 4. Case of microbiological proven Penicillium marneffei cerebral abscesses. (a, b) Multiple abscesses are noted at right frontal and
parietal white matter. One of the abscesses being T1 hypointense and T2 hyperintense is noted at the right thalamus. Involvement of deep
grey matter is not common in pyogenic abscess but is seen in fungal cerebral abscess. (c) Ring enhancement pattern of fungal abscess is
noted. (d, e) Diffusion-weighted imaging sequence and apparent diffusion coefficient map showing restricted diffusion of abscess wall at
the right thalamus. No restricted diffusion of the abscess cavity is seen. This feature suggests the underlying aetiology is more likely to be
fungal than pyogenic. (f) Hyperintensity of abscess cavity is seen on fluid-attenuated inversion recovery sequence.
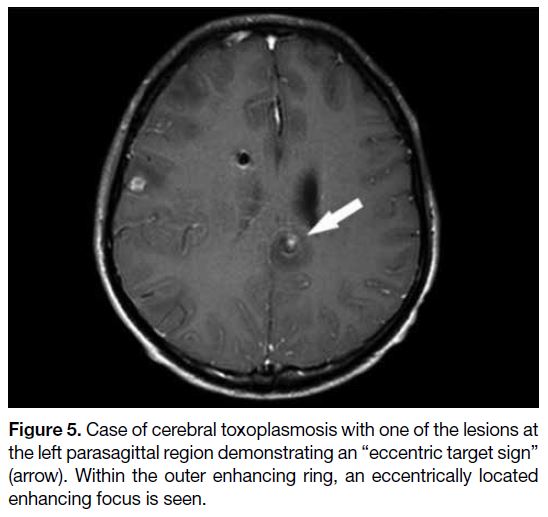
Cerebral Toxoplasmosis
Cerebral toxoplasmosis is a parasitic infection in the brain
with Toxoplasma gondii. It is the most common cause of brain abscesses among HIV-positive patients and is also
an AIDS-defining illness.[8] In immunocompetent people,
infection is often asymptomatic. Cause of infection is
most likely due to ingestion of food contaminated by cat
faeces that carry the oocytes of the parasites. On MRI,
cerebral toxoplasmosis usually appears as multiple small
abscesses at the grey-white junction, basal ganglia, and
thalami.[9] They are usually T1 isointense to hypointense
with variable T2 signal. No definite restricted diffusion
within the abscess cavity is seen. Ring enhancement
is often seen on contrast injection. The characteristic
“eccentric target sign” is highly specific for cerebral
toxoplasmosis but is only present in 30% of cases.[10] The
lesion has an inner eccentric enhancing core surrounded
by a hypointense zone and an outer peripherally
enhancing rim, overall giving an eccentric mural nodule
appearance (Figure 5).[11]
Figure 5. Case of cerebral toxoplasmosis with one of the lesions at
the left parasagittal region demonstrating an “eccentric target sign”
(arrow). Within the outer enhancing ring, an eccentrically located
enhancing focus is seen.
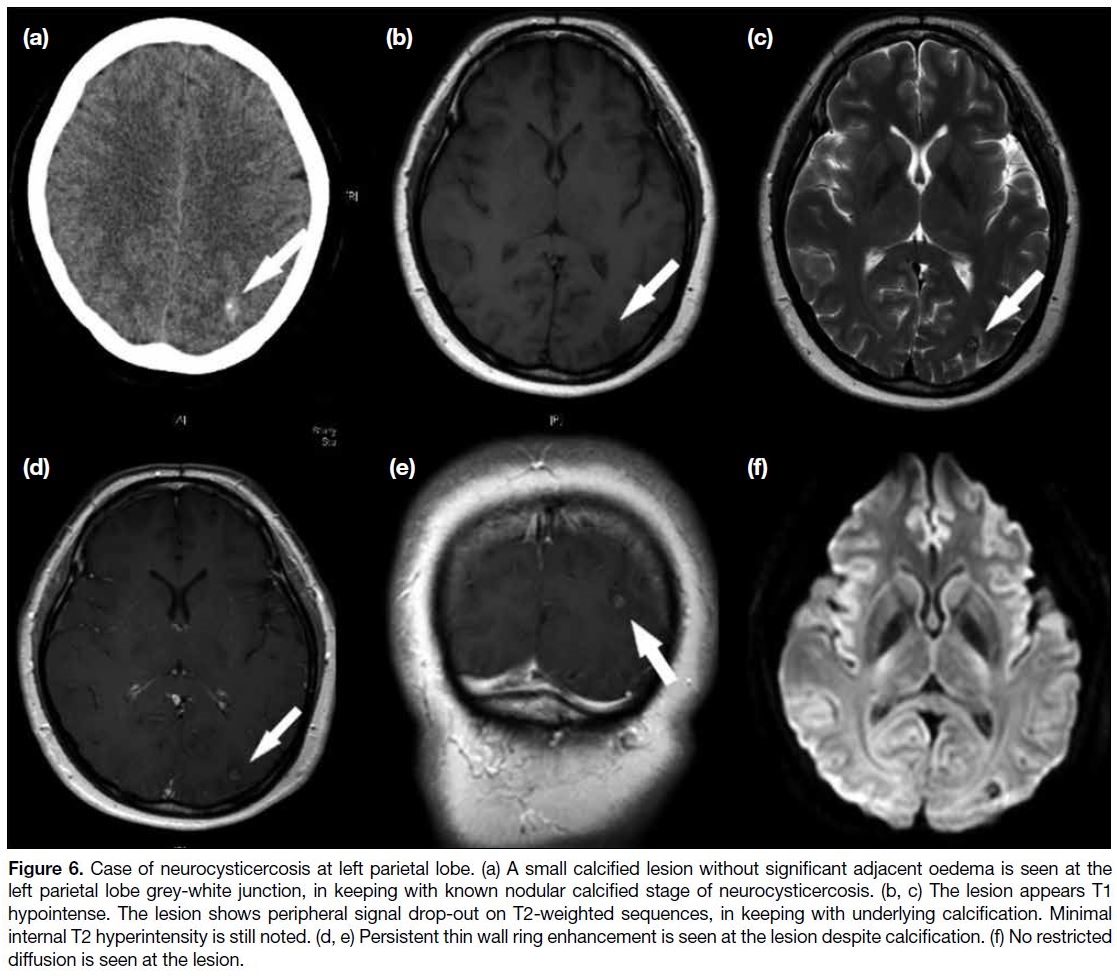
Neurocysticercosis
Neurocysticercosis is caused by CNS infection with the
pork tapeworm Taenia solium. The disease is endemic
in certain parts of Asia, Africa, and America. The most
common presentation in endemic areas is seizure. Other
presentations will depend on the site of involvement
in the brain. If the lesion is seen within the ventricular
system and causes obstruction to cerebrospinal fluid flow,
hydrocephalus may develop. The disease is spread by
ingestion of food contaminated with Taenia solium eggs.
Neurocysticercosis develops over four stages. During
the vesicular stage, the membrane of the parasite is intact
and the parasite is viable. In the colloidal vesicular stage,
the parasite dies and the membrane becomes leaky.
Significant adjacent oedema around different parasitic
lesions is seen during this stage. The granular nodular
stage is reached when the extent of oedema decreases.
All lesions ultimately become calcified with no more
adjacent oedema and the disease enters a nodular calcified
stage. MRI features of neurocysticercosis are essentially
thin-walled ring-enhancing lesions. The lesions can be
distributed at the subarachnoid space, brain parenchyma
especially grey-white junction, and the ventricles of
the brain. At the early stage, enhancing nodules may
be seen within the ring-enhancing lesions. However,
the most typical appearance of neurocysticercosis is
multiple calcified nodules with no significant oedema
in the final nodular calcified stage. Hypointensity
is seen on T2-weighted sequence due to underlying
calcification. Differing from dystrophic calcification of
brain, persistent ring enhancement can be noted despite
calcification of the lesion (Figure 6).[12]
Figure 6. Case of neurocysticercosis at left parietal lobe. (a) A small calcified lesion without significant adjacent oedema is seen at the
left parietal lobe grey-white junction, in keeping with known nodular calcified stage of neurocysticercosis. (b, c) The lesion appears T1
hypointense. The lesion shows peripheral signal drop-out on T2-weighted sequences, in keeping with underlying calcification. Minimal
internal T2 hyperintensity is still noted. (d, e) Persistent thin wall ring enhancement is seen at the lesion despite calcification. (f) No restricted
diffusion is seen at the lesion.
NEOPLASTIC CAUSES
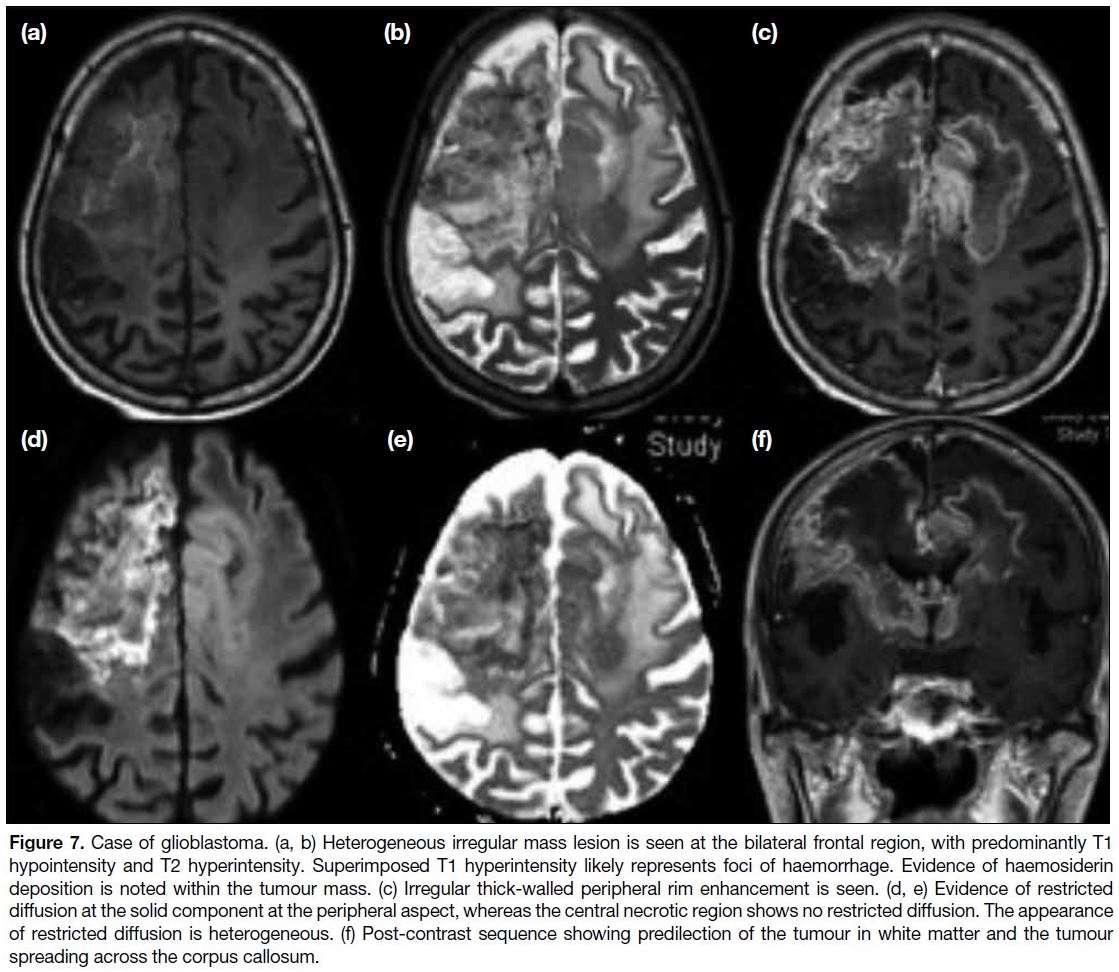
Glioblastoma
Glioblastoma is a high-grade astrocytoma and is the most common primary brain tumour in adults. It can
be classified as primary or secondary (arising from a
low-grade astrocytoma). Most primary glioblastomas
are isocitrate dehydrogenase wild-type whereas
secondary glioblastoma is more likely to have
isocitrate dehydrogenase–mutant status.[11] Up to 90%
of glioblastomas are primary and more commonly
seen in elderly patients. The tumour arises in cerebral
white matter and has a high tendency to spread across
the corpus callosum. Similar to most brain tumours, it
is T1 hypointense and T2 hyperintense, but the signals
are much more heterogeneous and the outline of the
lesion is more irregular. These signal changes may
alter in the presence of superimposed haemorrhage or
central necrosis. Susceptibility artefact may occur due
to haemorrhage and is often irregular. Focal restricted
diffusion may be seen within the lesion, although the
signal may not be as homogenous as that with pyogenic
abscess. Irregular ring enhancement is a feature of glioblastoma, often associated with a thick enhancing
rim (Figure 7). Prognosis of the disease is generally very
poor due to its fast growth and aggressive behaviour.
Figure 7. Case of glioblastoma. (a, b) Heterogeneous irregular mass lesion is seen at the bilateral frontal region, with predominantly T1
hypointensity and T2 hyperintensity. Superimposed T1 hyperintensity likely represents foci of haemorrhage. Evidence of haemosiderin
deposition is noted within the tumour mass. (c) Irregular thick-walled peripheral rim enhancement is seen. (d, e) Evidence of restricted
diffusion at the solid component at the peripheral aspect, whereas the central necrotic region shows no restricted diffusion. The appearance
of restricted diffusion is heterogeneous. (f) Post-contrast sequence showing predilection of the tumour in white matter and the tumour
spreading across the corpus callosum.
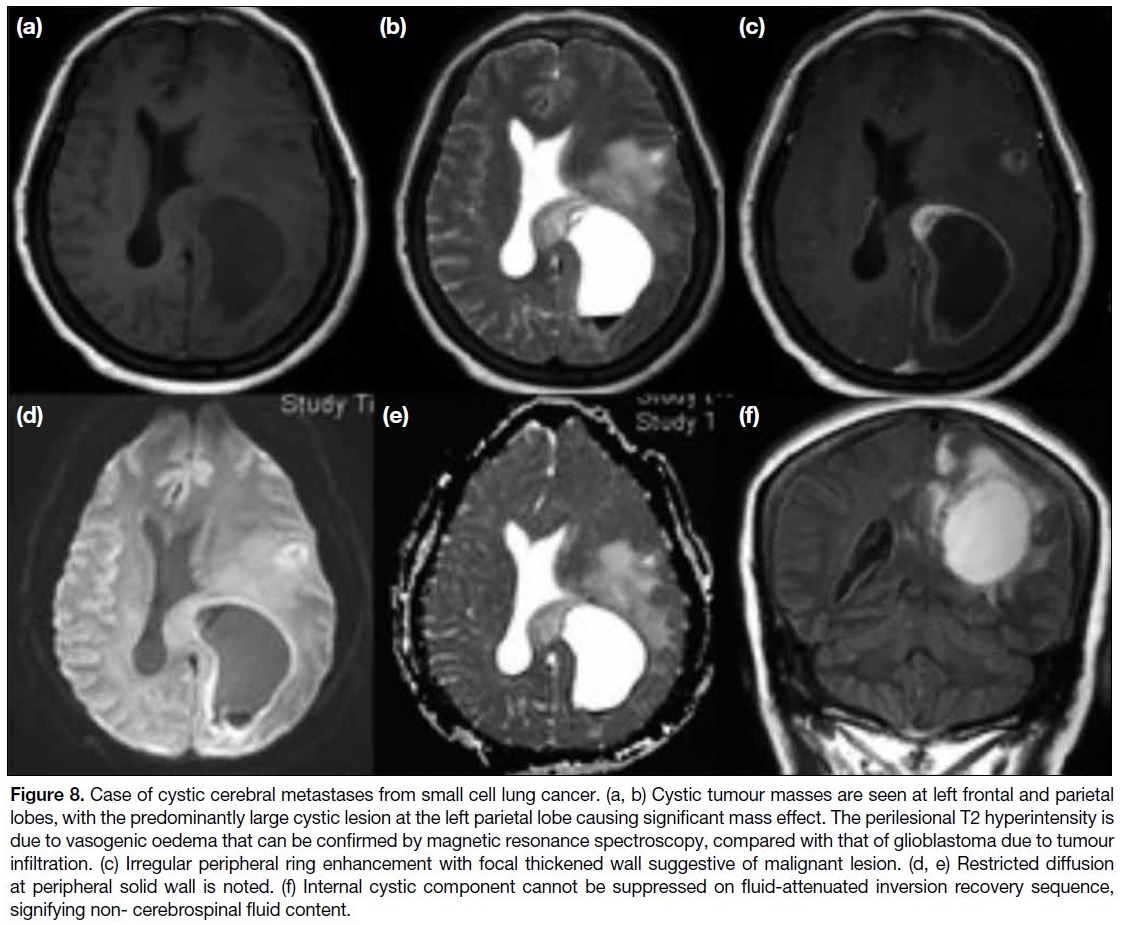
Brain Metastasis
Brain metastasis is a more common intracranial
malignancy than primary malignant brain tumour.
Common tumours that metastasise to the brain include
those of lung cancer, malignant melanoma, renal cell
carcinoma, breast cancer, and colorectal carcinoma.[13]
Brain metastasis can be solitary or multiple. Most
metastatic lesions are T1 hypointense and T2
hyperintense except for malignant melanoma, in which
the intrinsic melanin pigment will cause a reduction in
T1 relaxation time with a consequent T1 hyperintense
appearance.[14] When the tumour is complicated with
haemorrhage, T1 hyperintensity of the lesion will be noted. Enhancement can be uniform or ring-shaped
and the wall of ring-enhancing lesions is often thick
and irregular. Central necrosis may be seen within the
tumour, and no restricted diffusion can be seen within
the necrotic region (Figure 8).
Figure 8. Case of cystic cerebral metastases from small cell lung cancer. (a, b) Cystic tumour masses are seen at left frontal and parietal
lobes, with the predominantly large cystic lesion at the left parietal lobe causing significant mass effect. The perilesional T2 hyperintensity is
due to vasogenic oedema that can be confirmed by magnetic resonance spectroscopy, compared with that of glioblastoma due to tumour
infiltration. (c) Irregular peripheral ring enhancement with focal thickened wall suggestive of malignant lesion. (d, e) Restricted diffusion
at peripheral solid wall is noted. (f) Internal cystic component cannot be suppressed on fluid-attenuated inversion recovery sequence,
signifying non- cerebrospinal fluid content.
Differentiation between metastases and primary
glioblastoma may be difficult because the latter may also
present with multiple enhancing foci. For metastases,
they tend to involve the grey-white junction and rarely
spread along the corpus callosum unlike those of
glioblastoma. Moreover, if multiple enhancing tumours
are not connected by a single patch of T2/FLAIR
abnormality, they are more likely due to metastases.[15]
If the same characteristic is seen in glioblastoma, it is
termed multicentric glioblastoma and considered a rare
entity since it means there are multiple synchronous glioblastoma within the brain.[15] MR spectroscopy may
be useful to distinguish the two diseases by investigation
of the region of T2 hyperintensity around the ring of
enhancement. In metastases, the region of adjacent T2
hyperintensity often represents vasogenic oedema and
there will not be any increased choline-to-creatinine
ratio. In glioblastoma, the T2 hyperintensity might
represent non-enhancing tumour infiltration and will
be evidenced by increased choline-to-creatinine ratio at
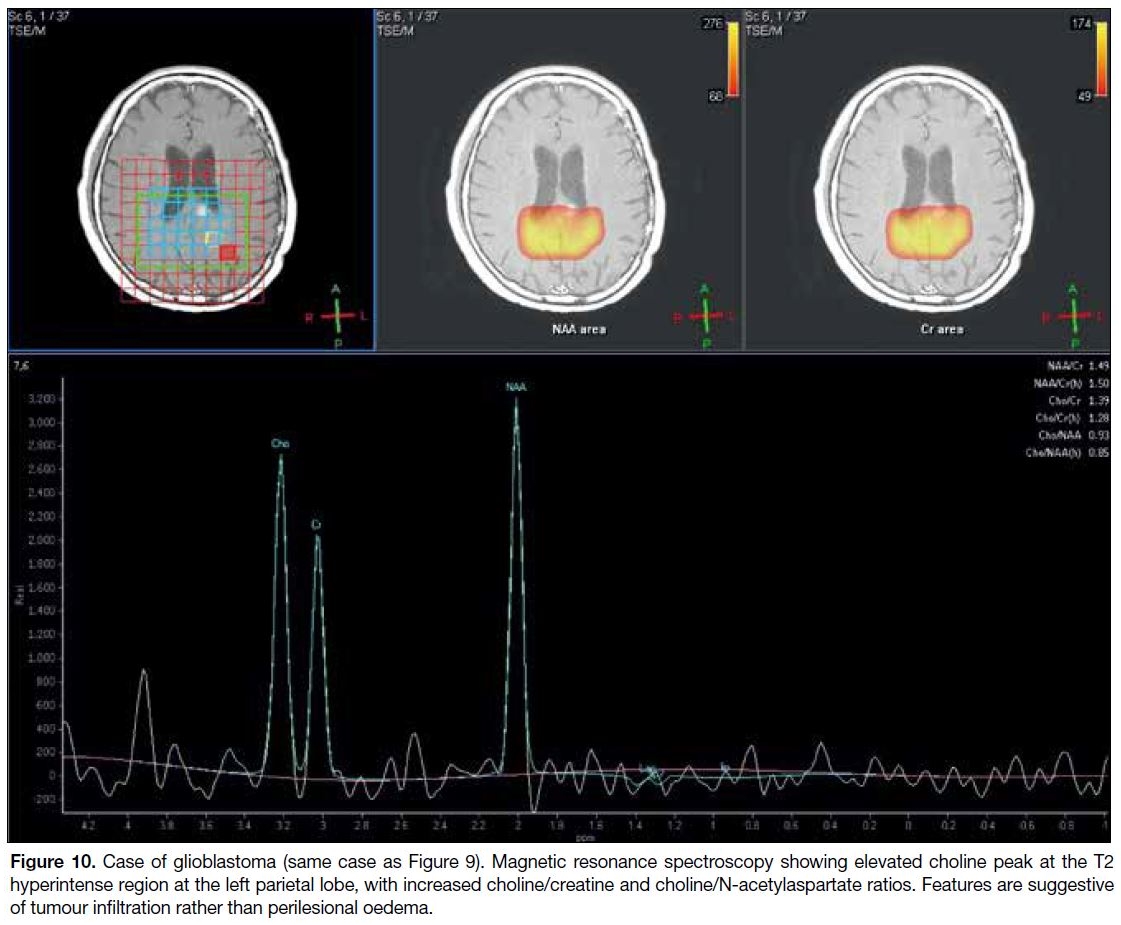
those regions (Figures 9 and 10).[16]
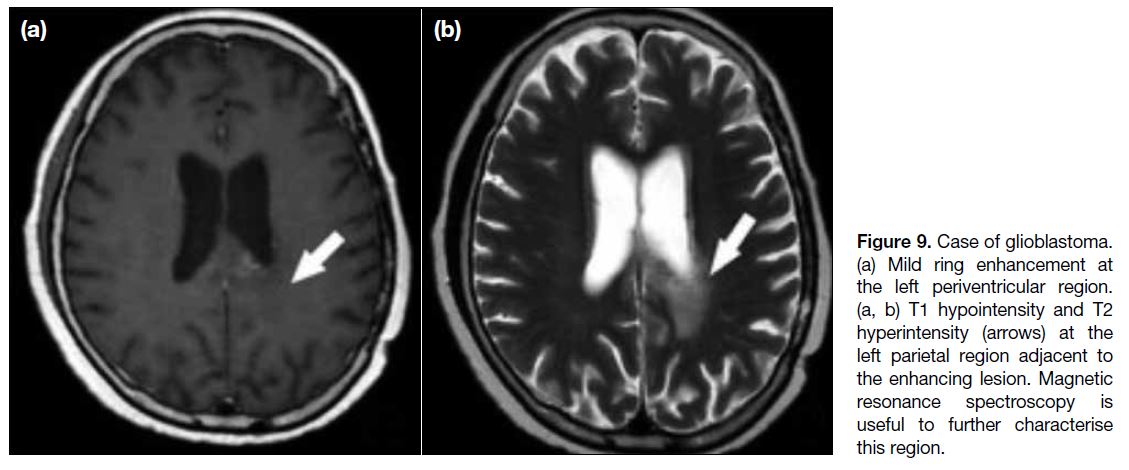
Figure 9. Case of glioblastoma.
(a) Mild ring enhancement at the left periventricular region. (a, b) T1 hypointensity and T2 hyperintensity (arrows) at the left parietal region adjacent to the enhancing lesion. Magnetic resonance spectroscopy is useful to further characterise this region.
Figure 10. Case of glioblastoma (same case as Figure 9). Magnetic resonance spectroscopy showing elevated choline peak at the T2
hyperintense region at the left parietal lobe, with increased choline/creatine and choline/N-acetylaspartate ratios. Features are suggestive
of tumour infiltration rather than perilesional oedema.
Primary Central Nervous System Lymphoma
Primary CNS lymphoma means there is no systemic
lymphomatous involvement when the disease is
diagnosed. Otherwise, it is just classified as secondary
intracranial involvement of lymphoma. The presentation
again depends on the location and size of the lesions.
One special characteristic of primary CNS lymphoma
is a predilection for supratentorial white matter.[17]
Similar to glioblastoma, it has a tendency to spread
across the corpus callosum. The disease can be seen in
immunocompetent and immunocompromised patients
although the appearance may be slightly different
depending on the patient’s immune status. On computed
tomography, it appears as a homogenous hyperdense
lesion with diffuse contrast enhancement. On MRI,
primary CNS lymphoma is typically T1 hypointense and
T2 hypointense/isointense with restricted diffusion. The
T2 hypointensity of primary CNS lymphoma makes it
a special characteristic as most intracranial masses are
T2 hyperintense. In immunocompetent patients, primary
CNS lymphoma usually demonstrates homogenous
enhancement with diffuse restricted diffusion (Figure 11). In immunocompromised patients, the enhancement pattern is more heterogeneous and the lesion will more
likely demonstrate ring enhancement.[17] Central necrosis
of tumour tends to occur in immunocompromised
patients, so focal T2 hyperintensity may be evident
within the lesions. A characteristic of MR spectroscopy
in primary CNS lymphoma is markedly elevated choline-to-creatinine ratio.[18]
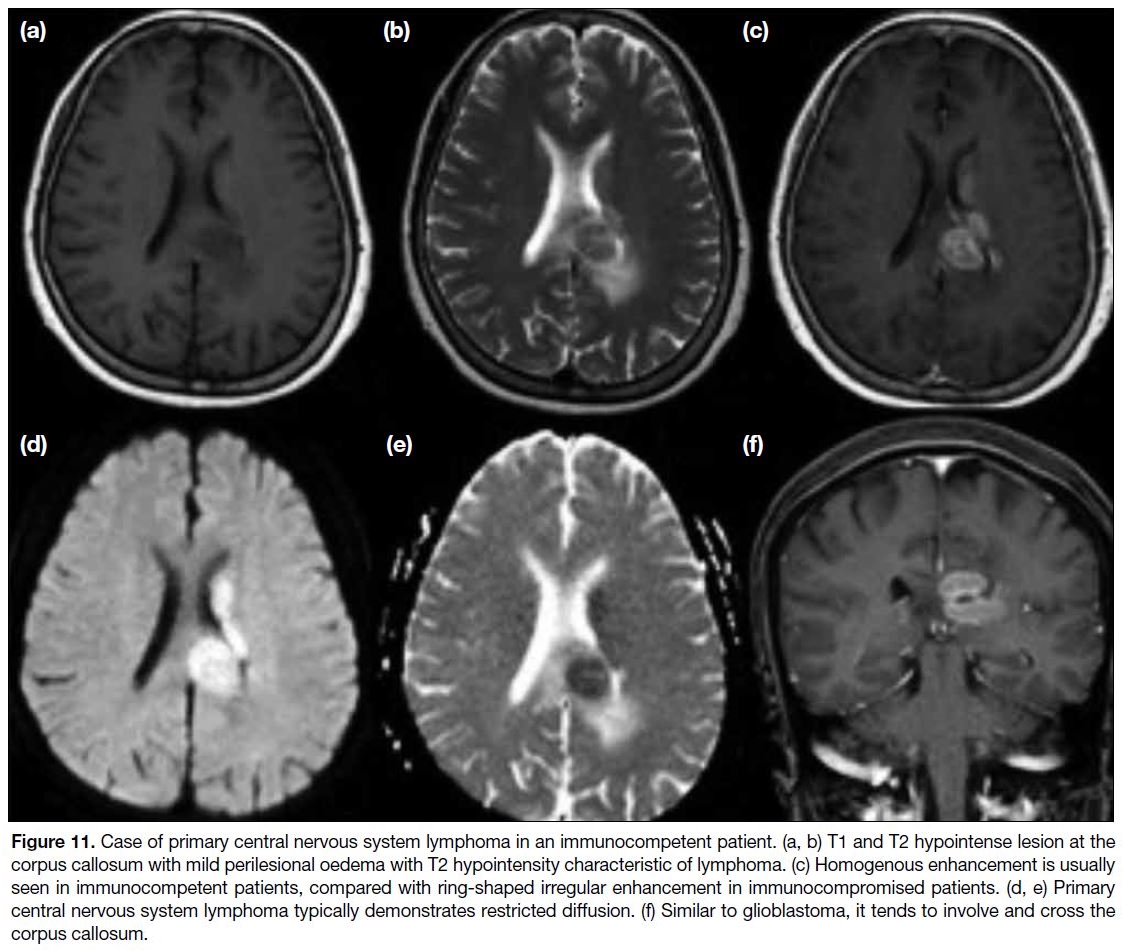
Figure 11. Case of primary central nervous system lymphoma in an immunocompetent patient. (a, b) T1 and T2 hypointense lesion at the
corpus callosum with mild perilesional oedema with T2 hypointensity characteristic of lymphoma. (c) Homogenous enhancement is usually
seen in immunocompetent patients, compared with ring-shaped irregular enhancement in immunocompromised patients. (d, e) Primary
central nervous system lymphoma typically demonstrates restricted diffusion. (f) Similar to glioblastoma, it tends to involve and cross the
corpus callosum.
POST-RADIATION CAUSE
Pseudoprogression and Cerebral Radiation
Necrosis
Brain tumours such as glioblastoma and brain metastases
often require radiation therapy. This often imposes
diagnostic challenges as post-radiation changes to
the tumour may simulate the appearance of residual
or recurrent tumour. Pseudoprogression and cerebral
radiation necrosis are both sequelae of radiation therapy of the brain. Pseudoprogression usually occurs
in the first 3 months following completion of brain
radiation but can occur up to 6 months post-treatment. It
appears as an enlarging irregular ring-enhancing lesion
mimicking disease progression whereas it actually
represents a change related to underlying cell death.
The border of the lesion with pseudoprogression might
have a “Swiss cheese” or “soap bubble” appearance.[19]
A few advanced MR sequences may contribute to
diagnosis of pseudoprogression. On MR perfusion
scan, pseudoprogression will show reduced cerebral
blood volume whereas tumour usually has increased
cerebral blood volume (Figure 12). MR spectroscopy of
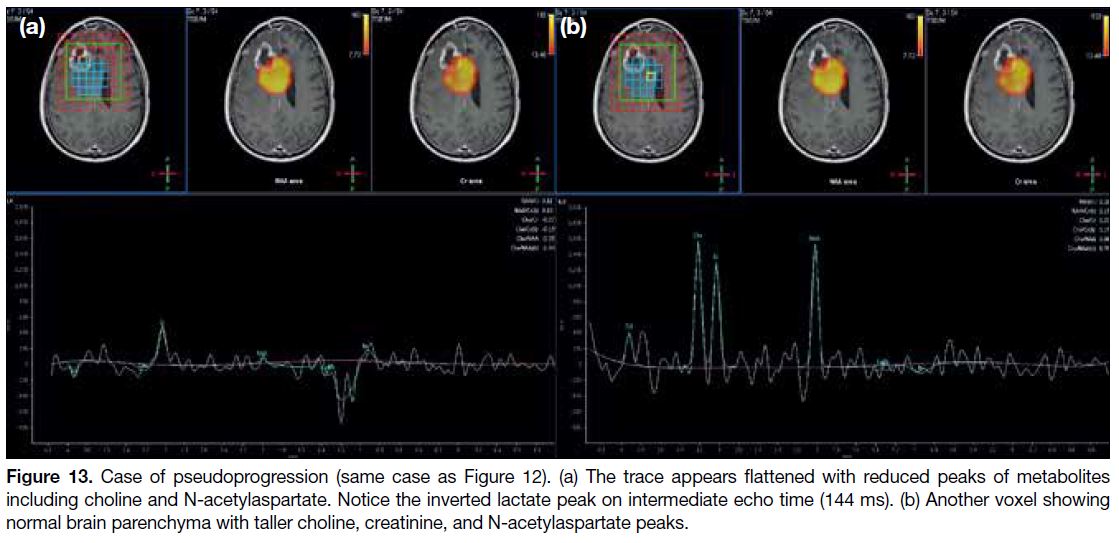
pseudoprogression often shows reduced metabolites and
increased lactate peak (Figure 13).[20] Stability or shrinkage
of the lesion through interval follow-up imaging can
also confirm that the lesion is related to post-radiation
changes. Cerebral radiation necrosis refers to a more
long-term effect of radiation therapy, usually beyond 6 months to years after treatment. The mass effect of
the lesion will be lost, unlike pseudoprogression. On the
other hand, the enhancing features, and findings on MR
spectroscopy and MR perfusion are similar to those of
pseudoprogression.
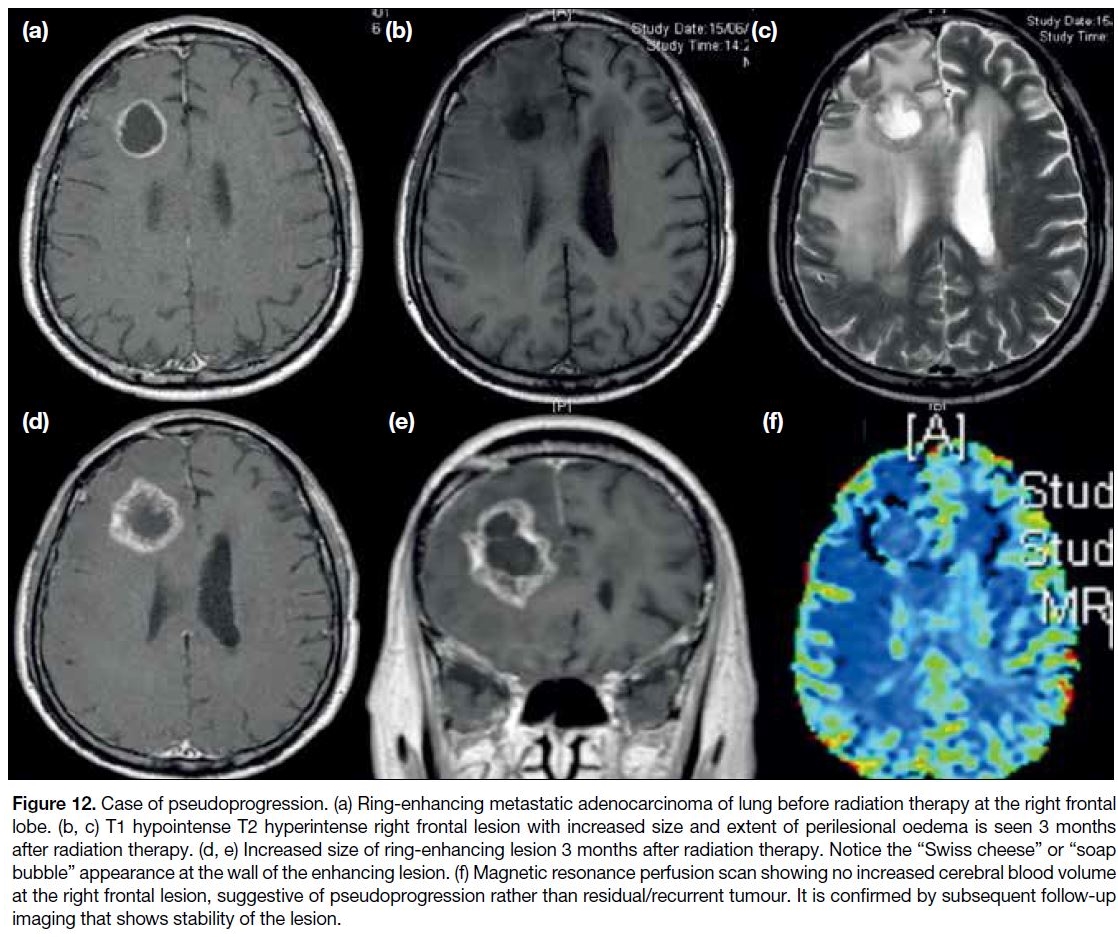
Figure 12. Case of pseudoprogression. (a) Ring-enhancing metastatic adenocarcinoma of lung before radiation therapy at the right frontal
lobe. (b, c) T1 hypointense T2 hyperintense right frontal lesion with increased size and extent of perilesional oedema is seen 3 months
after radiation therapy. (d, e) Increased size of ring-enhancing lesion 3 months after radiation therapy. Notice the “Swiss cheese” or “soap
bubble” appearance at the wall of the enhancing lesion. (f) Magnetic resonance perfusion scan showing no increased cerebral blood volume
at the right frontal lesion, suggestive of pseudoprogression rather than residual/recurrent tumour. It is confirmed by subsequent follow-up
imaging that shows stability of the lesion.
Figure 13. Case of pseudoprogression (same case as Figure 12). (a) The trace appears flattened with reduced peaks of metabolites
including choline and N-acetylaspartate. Notice the inverted lactate peak on intermediate echo time (144 ms). (b) Another voxel showing
normal brain parenchyma with taller choline, creatinine, and N-acetylaspartate peaks.
DEMYELINATING CAUSE
Multiple Sclerosis
One of the most common demyelinating diseases is
multiple sclerosis. The disease has several presentation
patterns. Involvement can be at the cerebrum,
cerebellum, brain stem, cranial nerves especially optic
nerves, and spinal cord. In general, multiple sclerosis is
predominantly seen in women, with a female-to-male
ratio up to 2:1.[21] MRI is the important imaging modality
in the radiological diagnosis of multiple sclerosis.
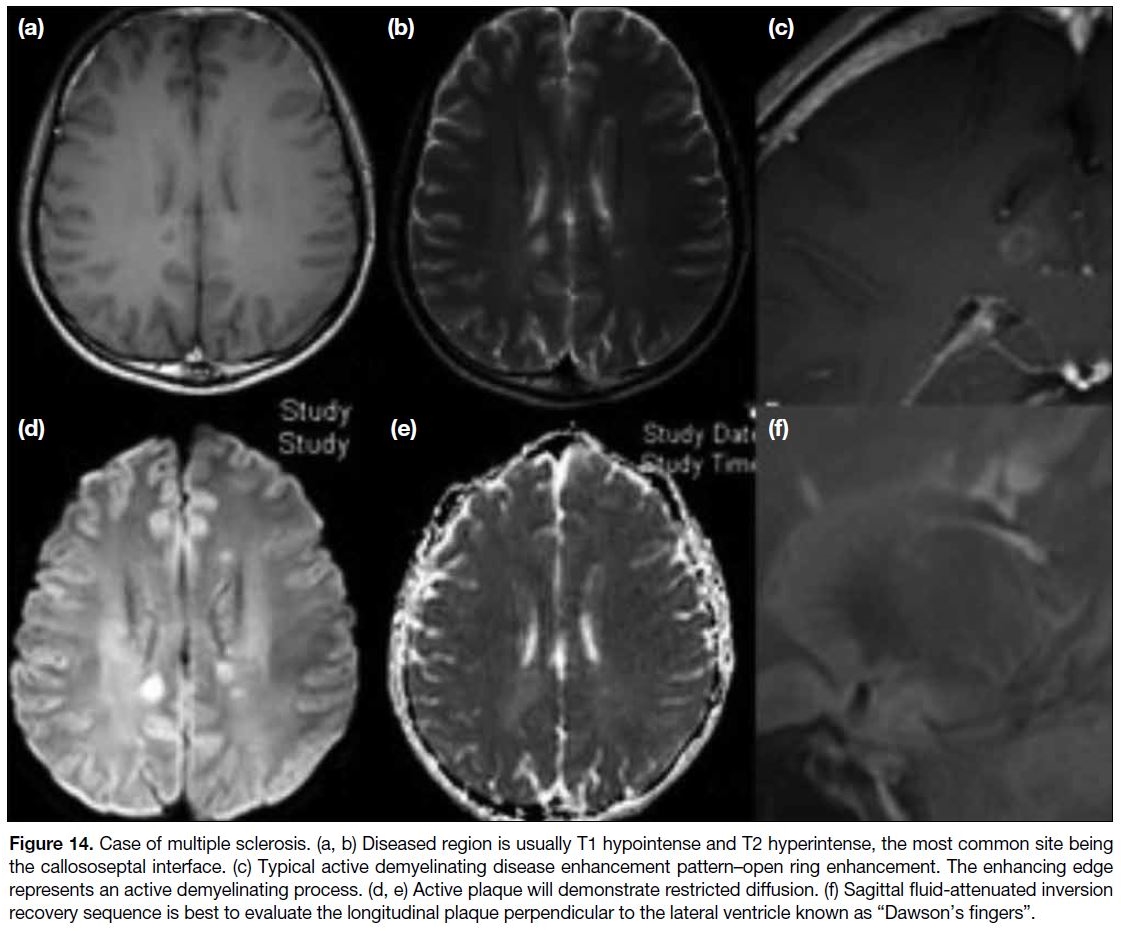
Diseased regions typically show foci of T1 hypointensity
and T2/FLAIR hyperintensity, with a predilection for
the callososeptal interface. They can slowly progress with longitudinal extension perpendicular to the lateral
ventricles, giving the typical “Dawson’s fingers”
appearance. Post-contrast and DWI sequences are useful
to detect active lesions/plaque. Sometimes these active
lesions may be quite large and mimic a mass lesion.
Active demyelinating lesions can be distinguished from
other malignant causes by the special ring-enhancing
pattern seen in the demyelinating disease known as “open
ring enhancement”,[22] which means there is incomplete
ring enhancement (Figure 14). The enhancing edge
represents an active demyelinating process in those
regions. This sign is rather specific for demyelination as
the underlying cause.[23]
Figure 14. Case of multiple sclerosis. (a, b) Diseased region is usually T1 hypointense and T2 hyperintense, the most common site being
the callososeptal interface. (c) Typical active demyelinating disease enhancement pattern–open ring enhancement. The enhancing edge
represents an active demyelinating process. (d, e) Active plaque will demonstrate restricted diffusion. (f) Sagittal fluid-attenuated inversion
recovery sequence is best to evaluate the longitudinal plaque perpendicular to the lateral ventricle known as “Dawson’s fingers“.
VASCULAR CAUSE
Haematoma
Hypertensive intracranial haemorrhage is the most
common cause of intracranial haemorrhage. It typically affects the basal ganglia, thalami, cerebellum, and pons.
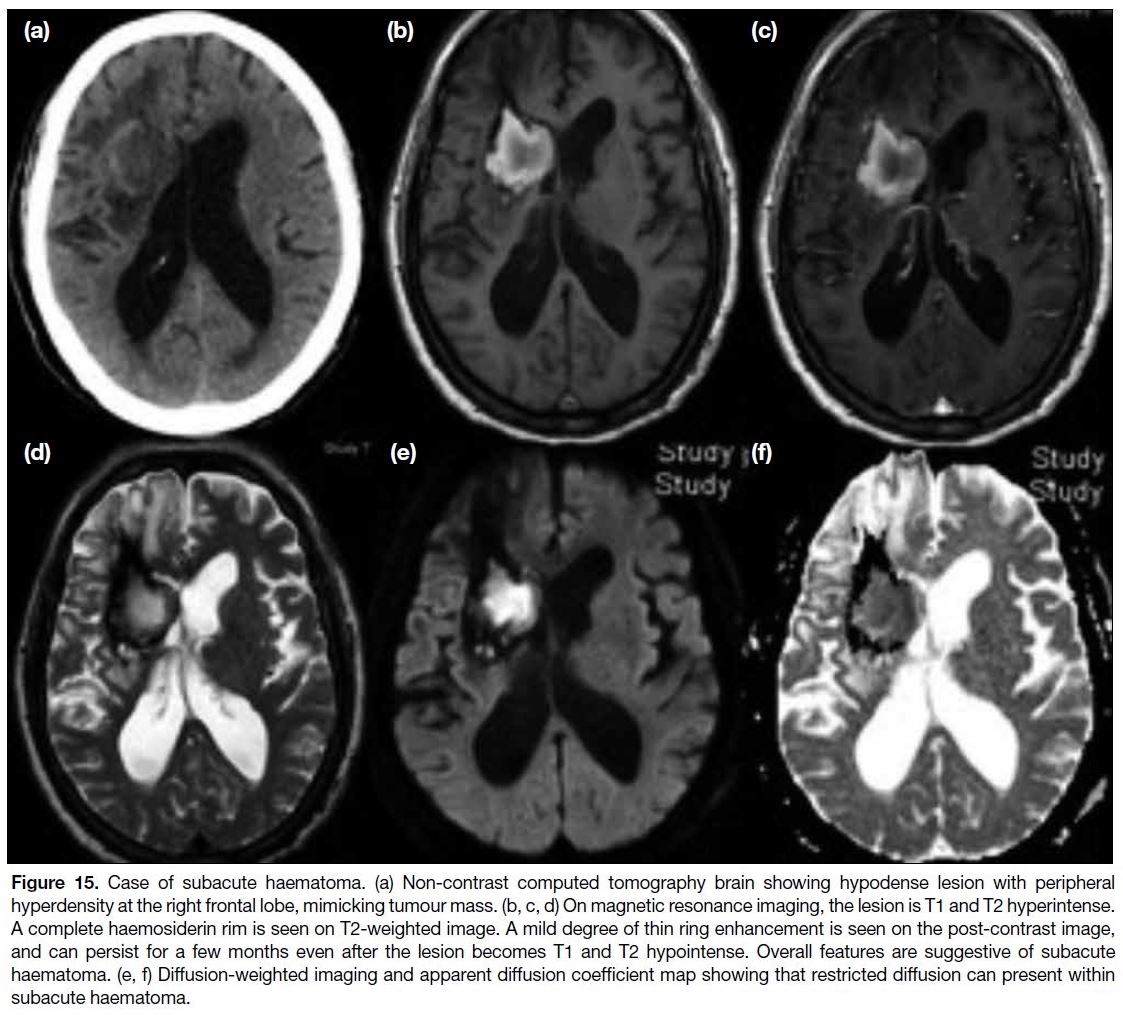
When the haematoma enters a subacute phase or early
chronic phase, thin peripheral enhancement around the
haematoma is often evident and may mimic tumour mass
lesion. Imaging characteristics of subacute haematoma
include T1 and T2 hyperintensity of the lesion. Also, the
peripheral ring enhancement should be thin. Perilesional
vasogenic oedema is usually not very significant. A
haemosiderin rim that is complete is often seen on T2-weighted image (Figure 15) and blooming artefact is
noted on DWI sequence. Interval follow-up scan can
exclude malignant lesions that will progress in size. If
MR perfusion is available, the cerebral blood flow will
decrease in case of subacute haematoma.[24]
Figure 15. Case of subacute haematoma. (a) Non-contrast computed tomography brain showing hypodense lesion with peripheral
hyperdensity at the right frontal lobe, mimicking tumour mass. (b, c, d) On magnetic resonance imaging, the lesion is T1 and T2 hyperintense.
A complete haemosiderin rim is seen on T2-weighted image. A mild degree of thin ring enhancement is seen on the post-contrast image,
and can persist for a few months even after the lesion becomes T1 and T2 hypointense. Overall features are suggestive of subacute
haematoma. (e, f) Diffusion-weighted imaging and apparent diffusion coefficient map showing that restricted diffusion can present within
subacute haematoma.
CONCLUSION
Cerebral ring-enhancing lesions are common features
in neuroradiological imaging. Although no imaging features are pathognomonic for certain disease entities,
a diagnosis can be reached or the differential diagnoses
narrowed through careful evaluation of a patient’s
clinical history, blood test results, certain radiological
features, and interval follow-up.
REFERENCES
1. Greenberg MS. Handbook of neurosurgery. New York: George
Thieme Verlag; 2006.
2. Haimes AB, Zimmerman RD, Morgello S, Weingarten K,
Becker RD, Jennis R, et al. MR imaging of brain abscesses. AJR
Am J Roentgenol. 1989;152:1073-85. Crossref
3. Whitener DR. Tuberculous brain abscess. Report of a case and
review of the literature. Arch Neurol. 1978;35:148-55. Crossref
4. Kim TK, Chang KH, Kim CJ, Goo JM, Kook MC, Han MH.
Intracranial tuberculoma: comparison of MR with pathologic
findings. AJNR Am J Neuroradiol. 1995;16:1903-8.
5. Bassiri-Jahromi S, Iravani K. Fungal brain abscess: report of three
cases and review of literature. Asian Pac J Trop Dis. 2014;4(Suppl 2):S854-9. Crossref
6. Gavito-Higuera J, Mullins CB, Ramos-Duran L, Olivas Chacon CI,
Hakim N, Palacios E. Fungal infections of the central nervous
system: a pictorial review. J Clin Imaging Sci. 2016;6:24. Crossref
7. Starkey J, Moritani T, Kirby P. MRI of CNS fungal infections:
review of aspergillosis to histoplasmosis and everything in between.
Clin Neuroradiol. 2014;24:217-30. Crossref
8. Johnson RT, Griffin JW, McArthur JC. Current therapy in
neurologic disease. 7th ed. Philadelphia: Mosby Inc; 2006.
9. Lee GT, Antelo F, Mlikotic AA. Best cases from the AFIP: cerebral
toxoplasmosis. Radiographics. 2009;29:1200-5. Crossref
10. Ramsay RG, Gerenia GK. CNS complications of AIDS: CT and
MRI findings. AJR Am J Roentgenol. 1988;151:449-54. Crossref
11. Kumar GG, Mahadevan A, Guruprasad AS, Kovoor JM,
Satishchandra P, Nath A, et al. Eccentric target sign in cerebral
toxoplasmosis: neuropathological correlate to the imaging feature.
J Magn Reson Imaging. 2010;31:1469-72. Crossref
12. Sheth TN, Pillon L, Keystone J, Kucharczyk W. Persistent MR
contrast enhancement of calcified neurocysticercosis lesions. AJNR
Am J Neuroradiol. 1998;19:79-82.
13. McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4(Suppl 4):S236-44. Crossref
14. Ginat DT, Meyers SP. Intracranial lesions with high signal intensity
on T1-weighted MR images: differential diagnosis. Radiographics.
2012;32:499-516. Crossref
15. Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura Lima A,
McCutcheon IE, et al. Multiple craniotomies in the management
of multifocal and multicentric glioblastoma. J Neurosurg.
2011;114:576-84. Crossref
16. Delorme S, Weber MA. Applications of MRS in the evaluation of
focal malignant brain lesions. Cancer Imaging. 2006;6:95-9. Crossref
17. Slone HW, Blake JJ, Shah R, Guttikonda S, Bourekas EC. CT and
MRI findings of intracranial lymphoma. AJR Am J Roentgenol.
2005;184:1679-85. Crossref
18. Haldorsen IS, Espeland A, Larsson EM. Central nervous system
lymphoma: characteristic findings on traditional and advanced
imaging. AJNR Am J Neuroradiol. 2011;32:984-92. Crossref
19. Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain
tumors. J Magn Reson Imaging. 2018;48:571-89. Crossref
20. Sawlani V, Taylor R, Rowley K, Redern R, Martin J, Poptani H.
Magnetic resonance spectroscopy for differentiating pseudo-progression
from true progression in GBM on concurrent
chemoradiotherapy. Neuroradiol J. 2013;25:575-86. Crossref
21. Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L,
Smirniotopoulos JG. White matter diseases with radiologic-pathologic
correlation. Radiographics. 2016;36:1426-47. Crossref
22. Given CA 2nd, Stevens BS, Lee C. The MRI appearance of
tumefactive demyelinating lesions. AJR Am J Roentgenol.
2004;182:195-9. Crossref
23. de Medeiros FC, de Albuquerque LA, Pittella JE, de Souza RB,
Gomes Neto AP, Christo PP. Open-ring enhancement in
pseudotumoral multiple sclerosis: important radiological aspect.
Case Rep Neurol Med. 2014;2014:951690. Crossref
24. Kamide T, Seki S, Suzuki K, Aoki T, Hirano K, Takahashi M, et al.
A chronic encapsulated intracerebral hematoma mimicking a brain
tumor: Findings on arterial spin labeling of MRI. Neuroradiol J.
2016;29:273-6. Crossref
| Attachment | Size |
|---|---|
| v24n1_Magnetic.pdf | 728.37 KB |