Comparison of Initial Risk Stratification Methods in Predicting Treatment Outcome in Differentiated Thyroid Cancer
ORIGINAL ARTICLE CME
Comparison of Initial Risk Stratification Methods in Predicting Treatment Outcome in Differentiated Thyroid Cancer
KM Wong, SI Soong, RMW Yeung
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Correspondence: Dr KM Wong, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong. Email: ka.ming.iris@gmail.com
Submitted: 22 Aug 2020; Accepted: 7 Dec 2020.
Contributors: All authors designed the study. KMW and SIS acquired the data, analysed the data, and drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by Hong Kong East Cluster Research Ethics Committee (Ref HKEC-2016-020). The patients were
treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed consent for all treatments and
procedures.
Abstract
Introduction
Differentiated thyroid cancer (DTC) is the commonest endocrine malignancy. With accurate risk
stratification, low-risk patients can be treated with less intensive treatment and follow-up. We reviewed outcomes
of DTC in our hospital and evaluated different staging methods in predicting survival.
Methods
This was a retrospective review including 321 patients with DTC treated in our hospital from 1994 to
2005. The cases were assessed for risk of recurrence using different risk stratification methods available at the time.
Disease-free survival (DFS) and overall survival (OS) of each method were analysed by comparing their Kaplan–
Meier plots with log rank tests. The Harrell C-index was used to evaluate the discriminative ability of different risk
stratification methods in identifying low- or high-risk cases.
Results
The 10-year DFS and OS were 90.9% and 93%, respectively. Comparing the C-indices, the European
Organisation for Research and Treatment of Cancer (EORTC) had the highest discriminative power for predicting
recurrence (C-index = 0.694, 95% confidence interval [CI] = 0.599-0.79) and OS (C-index = 0.825, 95% CI =
0.725-0.924). The MD Anderson Cancer Center (MDA) came in second for predicting recurrence (C-index = 0.669,
95% CI = 0.584-0.753) and OS (C-index = 0.769, 95% CI = 0.690-0.849). The commonly used American Joint
Committee on Cancer and the International Union Against Cancer/Tumour, Node, Metastasis system (AJCC/UICC
TNM) staging ranked third for predicting recurrence (C-index = 0.651, 95% CI = 0.561-0.740) and OS (C-index =
0.747, 95% CI = 0.630-0.863).
Conclusion
All risk stratification methods were reliable tools for initial risk stratification in DTC. We recommend
the use of AJCC/UICC TNM or MDA methods for their practicality.
Key Words: Disease-free survival; Mortality; Prognosis; Risk factors; Thyroid neoplasms
中文摘要
比較初步風險分層方法預測分化型甲狀腺癌的治療結果
黃嘉明、宋崧、楊美雲
引言
分化型甲狀腺癌(DTC)是最常見的內分泌惡性腫瘤。準確的風險分層可以對低危患者進行
較低強化治療和隨訪。我們回顧DTC的治療結果並評估以不同癌症分期預測存活率。
方法
這項回顧性研究納入1994年至2005年在我院接受治療的321例DTC患者。使用當時可用的不同
風險分層方法對這些病例的復發風險進行評估。 通過比較其Kaplan–Meier生存分析和對數秩檢驗來
分析每種方法的無病存活期(DFS)和總存活期(OS)。 Harrell-C指數用於評估在識別低風險或高
風險病例中不同風險分層方法的判別能力。
結果
10年無病存活率和總存活率分別為90.9%和93%。與C指數相比,歐洲癌症研究與
治療組織(EORTC)在預測復發率(C指數 = 0.694,95%置信區間 = 0.599-0.79)和總存活率
(C指數 = 0.825,95%置信區間 = 0.725-0.924)的鑑別力最高;其次為MD Anderson系統MDA
(預測復發率:C指數 = 0.669,95%置信區間 = 0.584-0.753;預測總存活率:C指數 = 0.769,
95%置信區間 = 0.690-0.849),以及常用的美國癌症聯合委員會和國際抗癌聯盟的TNM分期系統
AJCC/UICC TNM(預測復發率:C指數 = 0.651,95%置信區間 = 0.561-0.740;預測總存活率:
C指數 = 0.747,95%置信區間 = 0.630-0.863)。
結論
所有風險分層方法都是DTC中初始風險分層的可靠工具。就實用性而言,我們建議使用
AJCC/UICC TNM或MDA方法。
INTRODUCTION
Differentiated thyroid cancer (DTC) is the most common
endocrine malignancy and consists of papillary and
follicular subtypes.[1] DTC is the fifth most common
cancer among women in Hong Kong and statistics have
shown that the incidence of DTC was rising in the early
21st century globally, although studies, many in Asia,
have indicated that this is due to more screening, and not
due to an actual increase in disease rates.[2] [3]
Treatments for DTC commonly consist of surgical
excision, radioactive iodine (RAI) ablation, and thyroxine
suppressive therapy, as well as radiotherapy in some
cases of metastatic disease. For some patients with small
tumours <1 cm without lymph node involvement or high-risk
factors, active surveillance with ultrasonography may
also be considered.[4] With adequate treatment, patients
with early stages of DTC usually have excellent overall
survival (OS) with 5-year OS of approximately ≥95%.
Patients presenting in stage III or IV may have 5-year OS
of 40%.[5] Therefore, patients with low-risk disease may
be given less intensive treatment and follow-up so as to
minimise their exposure to radiation and medication. It is therefore important to have good risk stratification
methods to assist oncologists in risk stratification of
patients to allow formulation of an appropriate treatment
and follow-up plan.
In the past, several oncology centres or collaborations
across the globe devised risk stratification methods for
DTC. These include the American Joint Committee
on Cancer and the International Union Against Cancer
(AJCC/UICC), the Mayo Clinic, European Organisation
for Research and Treatment of Cancer (EORTC)
Thyroid Cancer Cooperative Group, the Memorial Sloan
Kettering (MSK) Cancer Center, the National Thyroid
Cancer Treatment Cooperative Study (NTCTCS) and
the MD Anderson Cancer Center (MDA). Given the
numerous risk stratification methods available and their
being mainly drawn from an overseas population, local
data and analysis will further aid clinicians to make
clinical decisions in local settings.
With increasing prevalence of DTC due to increased
screening, clinicians are anticipating a higher number
of patients with DTC in their clinics. Therefore, apart from the clinical applicability of each risk stratification
method, their practicality and reproducibility are also
essential factors for consideration of use in day-to-day
clinical practice. Some risk stratification methods
have included many clinicopathological factors for
categorisation while others have complicated scoring
systems, making them difficult to be adopted widely in
daily clinical practice.
In the present study, we aimed to review the treatment
outcome of DTC in our hospital and to evaluate the
efficacy, practicality, and applicability of different risk
stratification methods in a local setting.
METHODS
Study Design
This was a retrospective review of patients diagnosed
with DTC and treated and followed up in the Department
of Clinical Oncology of Pamela Youde Nethersole
Eastern Hospital from 1 January 1994 to 31 December
2005. Our study protocol was approved by the Hong
Kong East Cluster Ethics Committee and conducted in
accordance with the Declaration of Helsinki. Patient
consent was not required as it was a retrospective study
based on historical clinical records.
In our centre, a department protocol on management of
DTC is available for all doctors as a reference. In general,
patients with DTC were considered for adjuvant RAI if
they had undergone total thyroidectomy, unless they had
very low-risk disease with a favourable disease profile.
Otherwise, a RAI dosage of approximately 1100 to
3700 MBq was recommended for patients, depending on
their risk profile, such as tumour size and presence of
lymph node involvement. After ingestion of RAI, patients
would have whole-body scans within 1 week and a repeat
scan approximately 6 months later. Patients were started
on a thyroid-stimulating hormone–suppressive dose of
thyroxine unless contra-indicated, with dosage adjusted
later based on treatment response. If patients continued
to have RAI-avid residual disease on follow-up scans,
RAI was repeated. External beam radiotherapy of up
to 70 Gy over 35 fractions was considered for patients
with structural residual disease not amendable to further
surgery.[4]
Data Sources
Patient hospital records with primary diagnosis of
‘thyroid cancer’ during the above stated period were
retrieved from the Clinical Data Analysis and Reporting
System. Records including consultation notes, operative records, pathology reports, imaging reports including
ultrasonography and radioiodine scans, and blood results
were reviewed.
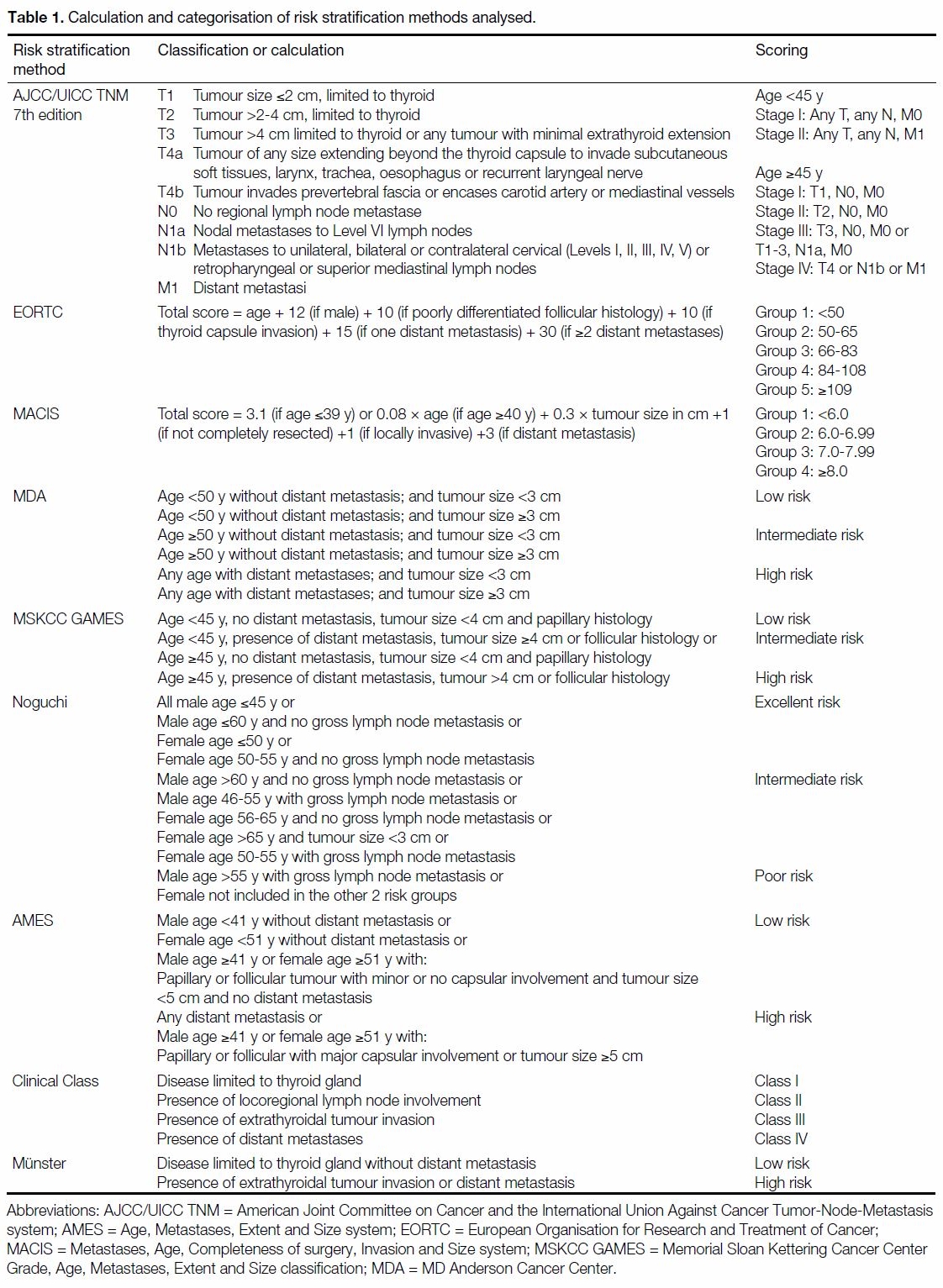
A MEDLINE search on DTC, staging and risk
stratification methods was done and found 17
documented risk stratification methods. They include the
AJCC/UICC Tumor-Node-Metastasis system (AJCC/UICC TNM) 7th edition, EORTC classification,[6] the
Metastases, Age, Completeness of surgery, Invasion
and Size system (MACIS),[7] the Age, Grade, Extent and
Size classification (AGES),[8] the Age, Metastases, Extent
and Size system (AMES),[9] the MSKCC Grade, Age,
Metastases, Extent and Size classification (MSKCC
GAMES),[10] the MDA,[11] the Clinical Class system
(Clinical Class),[12] the University of Münster system
(Münster),[13] the NTCTCS classification,[14] the Ohio State
University system (OSU),[15] the Noguchi classification
(Noguchi),[16] the University of Murcia system (Murcia),[17]
the Cancer Institute Hospital system (CIH),[18] the Ankara
Oncology Training and Research Hospital system
(Ankara),[19] the Sex, Age and Grade system (SAG),[20]
and the DNA, Age, Metastases, Extent and Size system
(DAMES).[21]
Of the 17 risk stratification systems, only nine could
be applied to our patients based on the patient data
available. The methods excluded and the various
reasons accountable for exclusion are: AGES (lack of
tumour grading data), OSU (lack of data on number
of intrathyroidal foci), SAG (lack of microscopic
description of nuclear atypia), NTCTCS (lack of tumour
grading data), CIH (lack of data on size of lymph nodes),
Ankara (lack of angioinvasion data), Murcia (lack of
histological subtype data), and DAMES (lack of DNA
ploidy data).
The calculations or categories used in the nine risk
stratification methods analysed in our study are
summarised in Table 1. Disease-free survival (DFS)
and OS were evaluated. DFS was defined as the date of
diagnosis to the date of relapse of DTC or death. OS was
defined as the date of diagnosis to the date of death from
any cause.
Table 1. Calculation and categorisation of risk stratification methods analysed.
Statistical Methods
DFS and OS of each risk classification system were
analysed by comparing their Kaplan–Meier plots with
log rank test. The Harrell’s C-index was used to evaluate
the discriminative ability of different risk stratification
methods in identifying low- or high-risk patients. A C-index of 1 implies that the risk stratification method
can perfectly select individuals with discordant events,
while a C-index of 0.5 shows that the risk stratification
method fails to show any discriminative ability.
C-index of >0.65 is considered an acceptable model for
predicting outcome. Statistical analysis was performed
using SPSS (Window version 22.0; IBM Corp, Armonk
[NY], United States) and R statistical software version
3.3.3.
RESULTS
A total of 321 patients were included in our study.
The median follow-up time was 143.3 months (range,
0.3-461.7). The percentage of patients with <1 year of
follow-up was 2.49%.
Demographics
Demographic distribution of our study population is
tabulated in Table 2. Females accounted for 81.3%
of the study population with median age 46 years.
A total of 79.4% of patients presented with thyroid
nodules and only 4% of patients reported symptoms
of thyrotoxicosis. Papillary carcinoma and follicular
carcinoma accounted for 91.6% and 8.4% of the
study cohort, respectively. About one-third (34%)
of our patients had multifocal disease confirmed on
histological examination.
Operative and Postoperative Treatment
A total of 94.4% of patients underwent total
thyroidectomy with 29.9% patients receiving planned
selective neck dissection according to the preoperative
lymph node status. Proportions of patients achieving
R0, R1 and R2 resections were 76%, 15%, and 2.2%,
respectively. Most of the patients (91.6%) received at
least one dose of RAI. For the first postoperative RAI,
the most commonly used dose was 80 mCi (93.9%)
according to our department protocol. On the first post-ablation
whole-body scan, 58.5% of patients showed
uptake over the thyroid bed only. On the subsequent
follow-up whole-body scan 6 months after RAI, 67.3%
of patients showed no significant uptake in the entire
body. Of the patients receiving postoperative RAI,
27.9% proceeded to receive a second-dose RAI while
8.9% further received a third RAI. Only about 5%
received postoperative external beam radiotherapy with
a median dose of 60 Gy (range, 50-64).
Survival Analysis
In our study, the 10-year and 15-year OS were 93% and
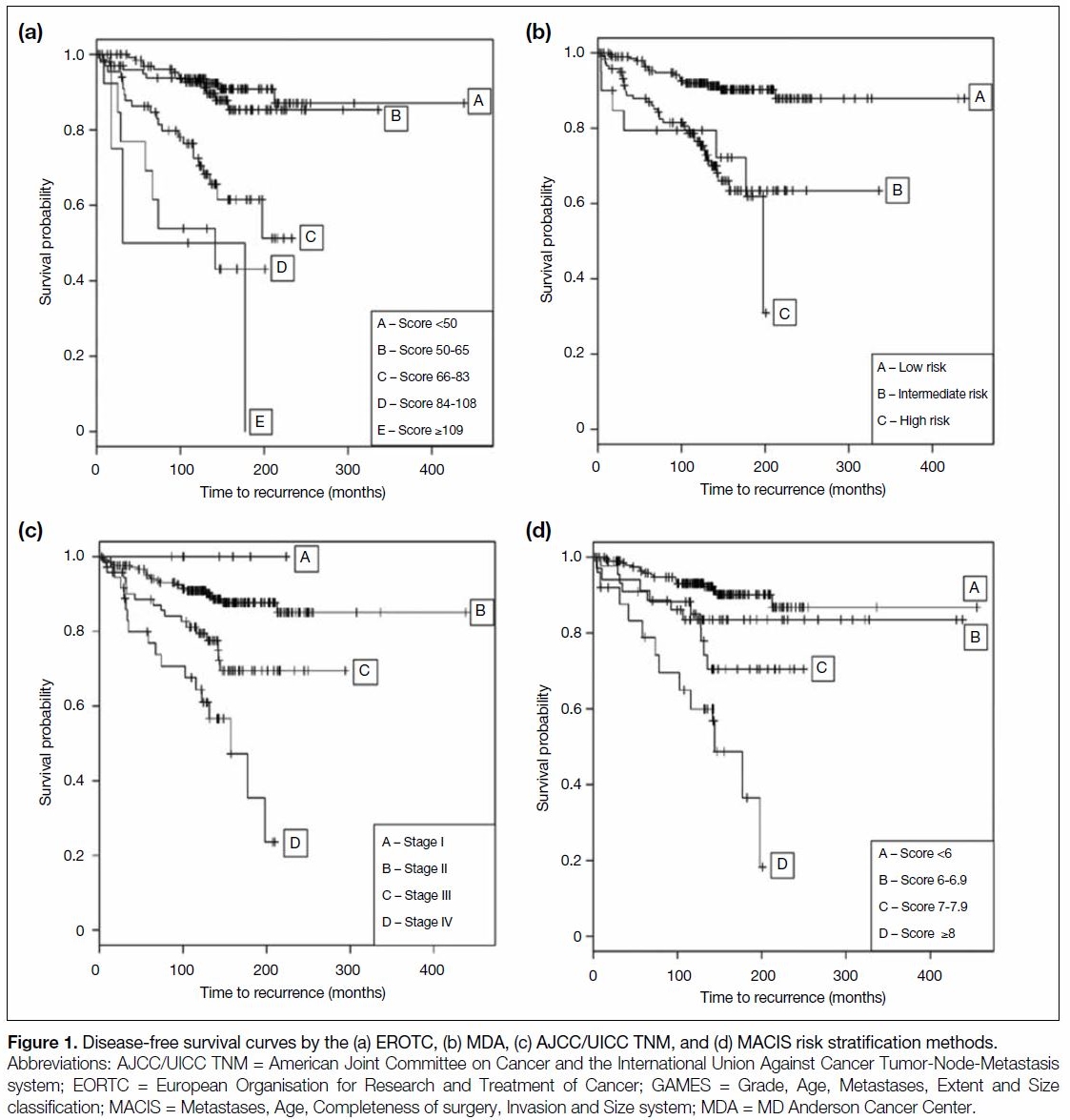
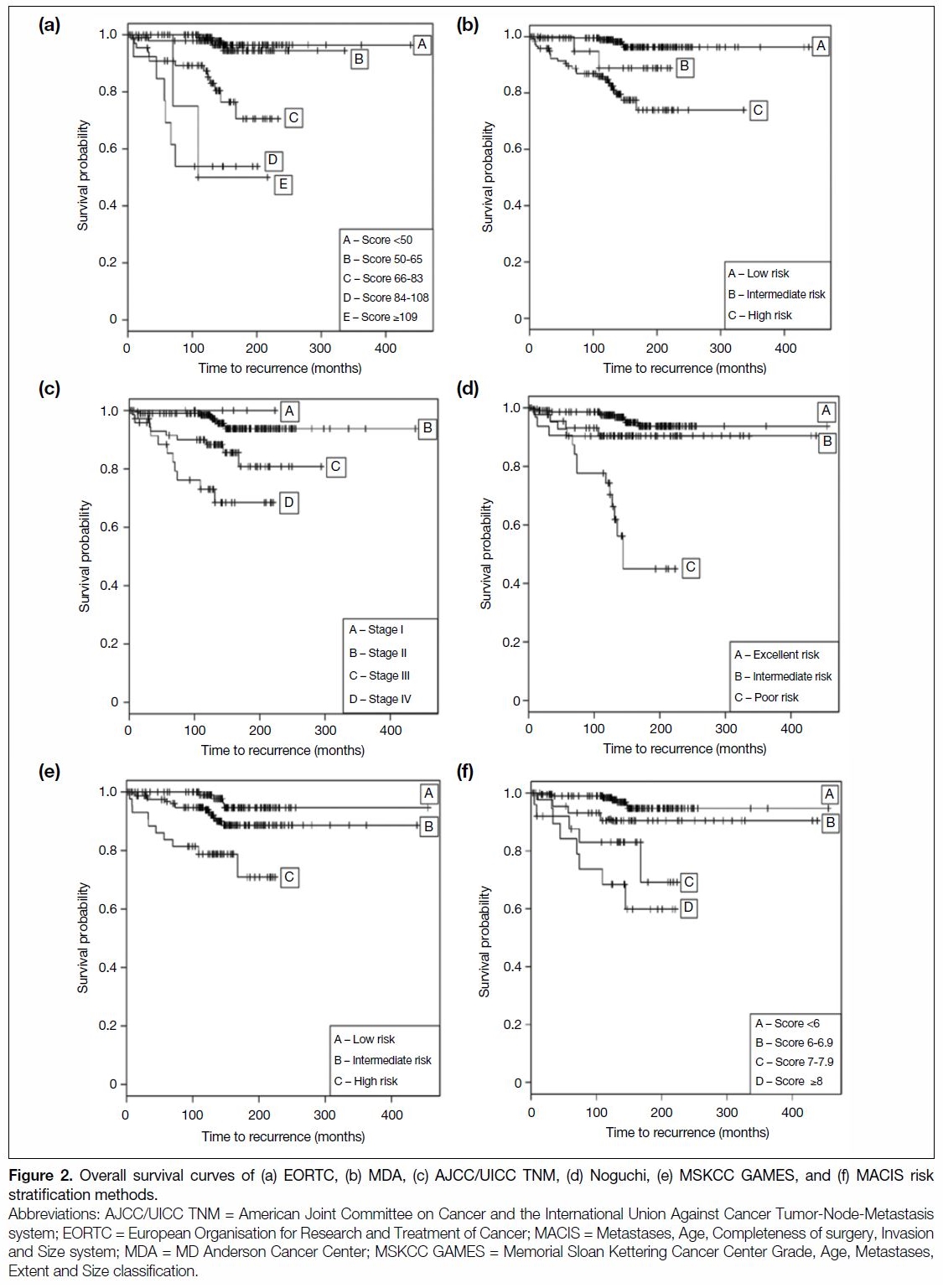
88.2%, respectively. The 10-year and 15-year DFS were 90.9% and 77%, respectively. The DFS Kaplan–Meier
curves for risk stratification methods with C-index of
≥0.65 are shown in Figure 1. The OS Kaplan–Meier
curves for risk stratification methods with C-index of
≥0.65 are shown in Figure 2.
Figure 1. Disease-free survival curves by the (a) EROTC, (b) MDA, (c) AJCC/UICC TNM, and (d) MACIS risk stratification methods.
Figure 2. Overall survival curves of (a) EORTC, (b) MDA, (c) AJCC/UICC TNM, (d) Noguchi, (e) MSKCC GAMES, and (f) MACIS risk stratification methods.
Comparison of Risk Stratification Methods
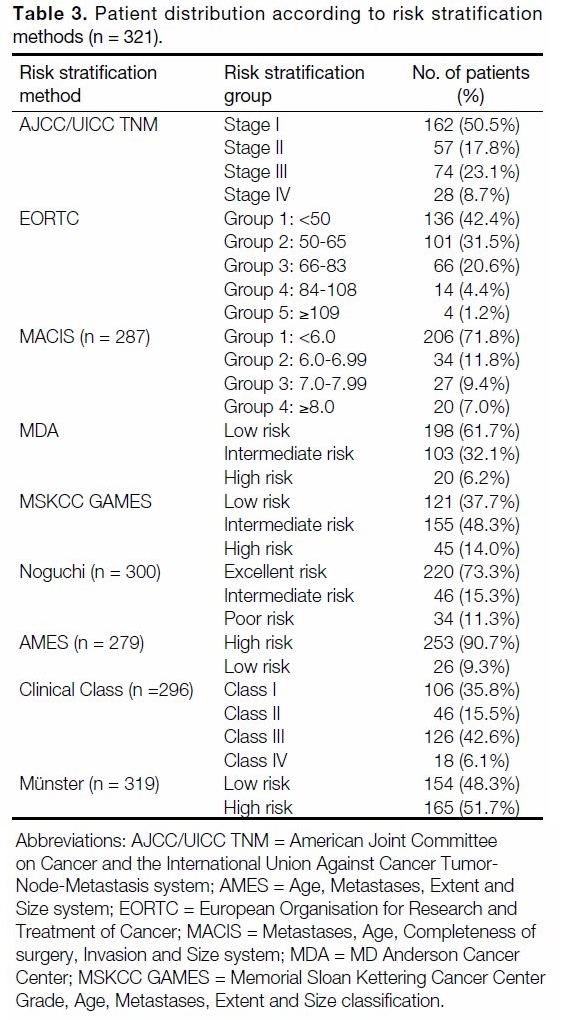
Distribution of patients in the different risk stratification methods is tabulated in Table 3. Table 4 shows the
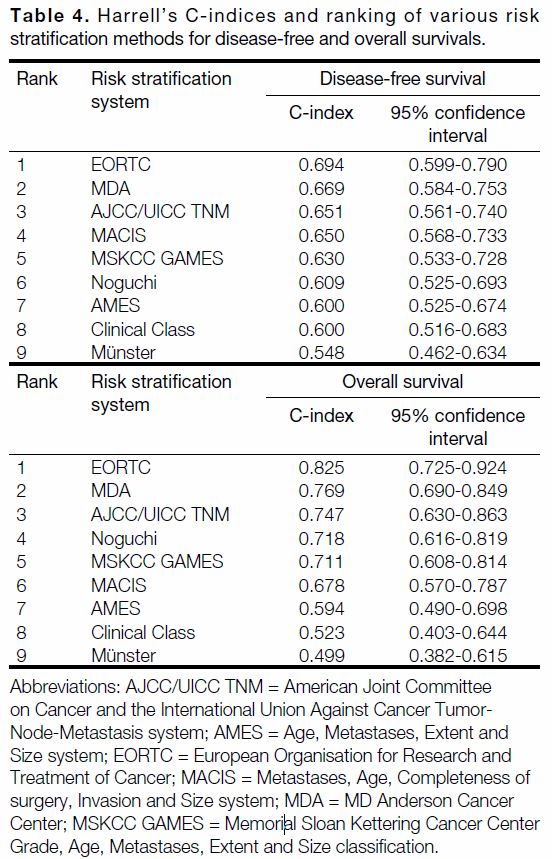
C-index of the risk stratification methods and their
rankings, in terms of DFS and OS, respectively. Comparing
the C-indices, EORTC had the highest discriminative
power for both predicting recurrence (C-index = 0.694,
95% confidence interval [CI] = 0.599-0.790) and OS
(C-index = 0.825, 95% CI = 0.725-0.924). MDA came in second in predicting recurrence (C-index = 0.669,
95% CI = 0.584-0.753) and OS (C-index = 0.769,
95% CI = 0.690-0.849). The AJCC/UICC TNM staging,
which we commonly used in our daily practice, ranked
third with a C-index of 0.651 (95% CI = 0.561-0.740)
for predicting recurrence and C-index of 0.747
(95% CI = 0.630-0.863) for predicting OS.
Table 3. Patient distribution according to risk stratification methods (n = 321).
Table 4. Harrell’s C-indices and ranking of various risk stratification methods for disease-free and overall survivals.
DISCUSSION
Precise clinicopathological staging is crucial in both
patient management and communication in doctor-patient
and doctor-doctor settings. A good staging
system provides reliable estimation of risk of recurrence
and disease-specific mortality for individual patients,
hence allowing clinicians to make evidence-based
decisions on the aggressiveness of adjuvant treatment,
intensity of follow-up, and for patient education and
counselling.[22] Moreover, a widely adopted staging
and risk stratification system can also allow clinicians
around the globe to communicate effectively, providing
a common language for medical discussion and research studies. An effective staging system should adequately
offer predictability, practicality, and reproducibility
in order to serve the multiple purposes mentioned
above.
Many systems have been proposed and studied for
risk stratification of DTC. Each system employs a
slightly different set of clinical and pathological factors.
Age and presence of metastases are well-recognised
prognostic factors in DTC and hence included in most
risk stratification methods.[23] [24] [25] [26] In our study, the EORTC,
MDA, and AJCC/UICC TNM methods came in the
first three positions in terms of discriminative ability to
differentiate low-risk from high-risk population, which
is in concordance with previous studies of this topic.[27] [28]
This may be accountable by the heavy weighting given
to age or the presence of distant metastases in these
systems. For instance, in the EORTC calculation, distant metastases give the highest contributing score of 15 to
30 depending on the number of metastases. Meanwhile,
for AJCC/UICC TNM staging method, all patients aged
<45 years belong to stage I or II regardless of tumour
size, local extent, or nodal involvement. For the MDA
method, age and presence of distant metastases are
the only consideration factors, eliminating other less
influential clinicopathological factors included in other
systems.
Direct comparison of the different risk stratification
methods can be difficult as each method includes
different histologies of DTC. All histologies of thyroid
cancer were included in the AJCC/UICC TNM and
EORTC systems, while only papillary thyroid cancer
is included in the MACIS, Clinical Class, and Noguchi
methods. Both papillary and follicular thyroid cancers
are included in the MDA, AMES, MSKCC GAMES,
and Münster methods. As papillary, follicular, medullary
and anaplastic thyroid cancer each has a distinct disease
behaviour and prognostic curve, the survival data
across two or more histology types are more difficult
to be interpreted and compared directly with data
from other studies. We recognise that medullary and
anaplastic thyroid cancers are rare disease entities, and
it is challenging to recruit adequate number of cases for
reliable analysis.
Practicality and reproducibility are essential
consideration factors in everyday clinical practice. Some
risk stratification systems involve a more complicated
calculation, allowing different clinicopathological factors
to have different weighting on the risk stratification
outcome. For instance, EORTC assigns a score to each
clinicopathological factor (eg, 12 for male sex, 15 for one
distant metastasis) and requires the sum of the scores.
Another example is in MACIS, where clinicians need
to perform multiplications and summation of various
clinicopathological factors (eg, multiplying age by
0.08 for patients aged ≥40 years, multiplying tumour size
in cm by 0.3). These may render certain risk stratification
methods less convenient to be applied in day-to-day
practice. Therefore, in the aspect of practicality, the
AJCC/UICC TNM and MDA systems appear more
intuitive and clinician-friendly for interpretation and
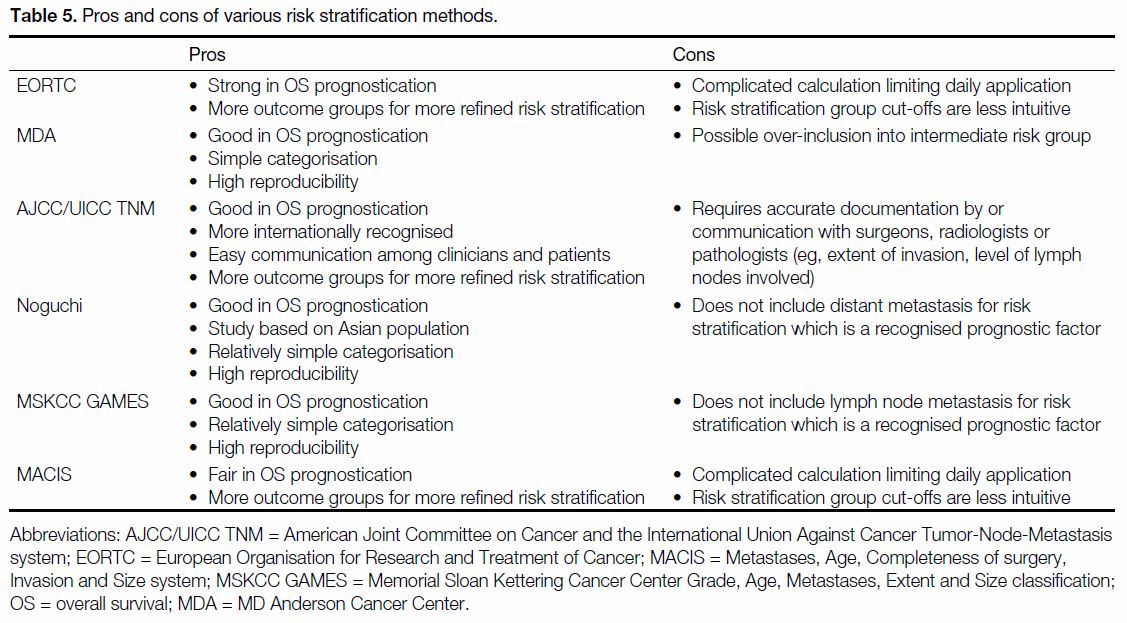
patient counselling. The pros and cons of each risk
stratification method with a good discriminative power
are listed in Table 5.
Table 5. Pros and cons of various risk stratification methods.
Several risk stratification methods have not been
included in our study as certain required variables are
not routinely available in our pathology reports. Some of
these pathological characteristics include size of lymph
nodes, nuclear atypias, and DNA ploidy. According
to the thyroid cancer structured reporting protocol by the International Collaboration on Cancer Reporting,
certain parameters, such as exact number of tumour
foci, size of lymph nodes, and tumour grading are
not required to be included in the pathology reports.[29]
These parameters have only been reported in limited
studies to be correlated with prognostication. A
prospective study in collaboration with pathologists
will be needed if these parameters are to be investigated
in the future.
Limitations
Limitations in our study include the inherent bias in its
retrospective design and the lack of complete pathological
data required for all risk stratification methods. The long
follow-up time in this study implies that there may have
been changes in practice, especially with the emerging
utility of thyroglobulin to guide clinical management. In
addition, further analysis with the newer version of the
AJCC/UICC TNM staging system would elucidate its
applicability in our local population.
Building on the basis of initial risk stratification, the role
of dynamic risk stratification is also coming to light in the
current era.[30] [31] We recognise that initial staging is only
the beginning of the risk stratification process. A more
tailor-made risk stratification specific to each individual
patient will require further clinical information on
follow-up, such as postoperative serum thyroglobulin
and post-RAI scan findings.[31] [32] [33] [34] With the increasing
availability of molecular analysis in the modern era,
certain molecular markers such as BRAF or RET may
also be factored in for more accurate risk stratification.[35] [36]
Therefore, although both AJCC/UICC TNM and MDA
can offer a reliable and convenient means for initial risk
stratification, we recommend that it should be coupled
with the American Thyroid Association risk stratification
system and further dynamic risk stratification (based
on presence of biochemical or structural evidence of
relapse over time) to best estimate an individual patient’s
survival and risk of recurrence.
CONCLUSION
AJCC/UICC TNM, EORTC, and MDA risk stratification
methods are all reliable tools for initial risk stratification
in DTC. We recommend the use of AJCC/UICC TNM
staging in daily practice for its practicality and global
recognition, in combination with the American Thyroid
Association and dynamic risk stratification to best predict
individualised risk of recurrence and survival in patients
with DTC.
REFERENCES
1. Figge JJ. Epidemiology of thyroid cancer. In: Wartofsky L, Van Nostrand D, editors. Thyroid Cancer. Washington: Humana
Press; 2006: p 9-13. Crossref
2. Hong Kong Cancer Registry, Hospital Authority. Thyroid Cancer
in 2018. Available from: https://www3.ha.org.hk/cancereg/pdf/
factsheet/2018/thyroid_2018.pdf. Accessed 17 Feb 2021.
3. Powers AE, Marcadis AR, Lee M, Morris LG, Marti JL. Changes
in trends in thyroid cancer incidence in the United States, 1992 to
2016. JAMA. 2019;322:2440-1. Crossref
4. National Comprehensive Cancer Network Clinical Practice
Guidelines in Oncology Thyroid Carcinoma Version 3.2020.
https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
Accessed 17 Feb 2021.
5. American Joint Committee on Cancer. AJCC Cancer Staging
Manual Seventh Edition 2010. Available from: http://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%.... Accessed 1 Aug 2020.
6. Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA,
et al. A prognostic index for thyroid carcinoma. A study of the
E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer.
1979;15:1033-41. Crossref
7. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS.
Predicting outcome in papillary thyroid carcinoma: development
of a reliable prognostic scoring system in a cohort of 1779 patients
surgically treated at one institution during 1940 through 1989.
Surgery. 1993;114:1050-7.
8. Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral
lobectomy versus bilateral lobar resection in papillary thyroid
carcinoma: a retrospective analysis of surgical outcome using a
novel prognostic scoring system. Surgery. 1987;102:1088-95.
9. Cady B, Rossi R. An expanded view of risk-group definition in
differentiated thyroid carcinoma. Surgery. 1988;104:947-53.
10. Shaha AR, Loree TR, Shah JP. Intermediate-risk group for
differentiated carcinoma of thyroid. Surgery. 1994;116:1036-40.
11. Beenken S, Roye D, Weiss H, Sellers M, Urist M, Diethelm A,
et al. Extent of surgery for intermediate-risk well-differentiated
thyroid cancer. Am J Surg. 2000;179:51-6. Crossref
12. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural
history, treatment, and course of papillary thyroid carcinoma. J
Clin Endocrinol Metab. 1990;71:414-24. Crossref
13. Lerch H, Schober O, Kuwert T, Saur HB. Survival of differentiated
thyroid carcinoma studied in 500 patients. J Clin Oncol.
1997;15:2067-75. Crossref
14. Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST,
Cooper DS, et al. Prospective multicenter study of thyroid
carcinoma treatment: initial analysis of staging and outcome.
National Thyroid Cancer Treatment Cooperative Study Registry
Group. Cancer. 1998;83:1012-21. Crossref
15. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical
and medical therapy on papillary and follicular thyroid cancer. Am
J Med. 1994;97:418-28. Crossref
16. Noguchi S, Murakami N, Kawamoto H. Classification of
papillary cancer of the thyroid based on prognosis. World J Surg.
1994;18:552-7. Crossref
17. Sebastian SO, Gonzalez JM, Paricio PP, Perez JS, Flores DP,
Madrona AP, et al. Papillary thyroid carcinoma: prognostic
index for survival including the histological variety. Arch Surg.
2000;135:272-7. Crossref
18. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel
classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases
and reclassification during the follow-up period. Surgery.
2004;135:139-48. Crossref
19. Yildirim E. A model for predicting outcomes in patients with
differentiated thyroid cancer and model performance in comparison
with other classification systems. J Am Coll Surg. 2005;200:378-92. Crossref
20. Akslen LA. Prognostic importance of histologic grading in papillary
thyroid carcinoma. Cancer. 1993;72:2680-5. Crossref
21. Pasieka JL, Zedenius J, Auer G, Grimelius L, Höög A, Lundell G,
et al. Addition of nuclear DNA content to the AMES risk-group
classification for papillary thyroid cancer. Surgery. 1992;112:1154-
9.
22. Tuttle RM, Alzahrani AS. Risk stratification in differentiated
thyroid cancer: from detection to final follow-up. J Clin Endocrinol
Metab. 2019;104:4087-100. Crossref
23. Bischoff LA, Curry J, Ahmed I, Pribitkin E, Miller JL. Is above age
45 appropriate for upstaging well-differentiated papillary thyroid
cancer? Endocr Pract. 2013;19:995-7. Crossref
24. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A,
et al. Survival from differentiated thyroid cancer: what has age got
to do with it? Thyroid. 2015;25:1106-14. Crossref
25. Casara D, Rubello D, Saladini G, Masarotto G, Favero A, Girelli ME,
et al. Different features of pulmonary metastases in differentiated
thyroid cancer: natural history and multivariate statistical analysis
of prognostic variables. J Nucl Med. 1993;34:1626-31.
26. Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H,
Nason RW, Pathak KA. The age factor in survival of a population
cohort of well-differentiated thyroid cancer. Endocr Connect.
2013;2:154-60. Crossref
27. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems
for papillary thyroid carcinoma: a review and comparison. Ann
Surg. 2007;245:366-78. Crossref
28. Lang BH, Chow SM, Lo CY, Law SC, Lam KY. Staging systems
for papillary thyroid carcinoma: a study of 2 tertiary referral centers.
Ann Surg. 2007;246:114-21. Crossref
29. International Collaboration on Cancer Reporting. Thyroid Cancer
Structured Reporting Protocol. 2nd edition. 2020. Available from:
https://www.rcpa.edu.au/getattachment/d2f937ce-5025-402b-8eb1-84e580f59f.... Accessed 1 Aug 2020.
30. Tuttle RM, Leboeuf R, Shaha AR. Medical management of thyroid
cancer: a risk adapted approach. J Surg Oncol. 2008;97:712-6. Crossref
31. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al.
Estimating risk of recurrence in differentiated thyroid cancer after
total thyroidectomy and radioactive iodine remnant ablation: using
response to therapy variables to modify the initial risk estimates
predicted by the new American Thyroid Association staging system.
Thyroid. 2010;20:1341-9. Crossref
32. Giovanella L, Castellana M, Trimboli P. Unstimulated high-sensitive
thyroglobulin is a powerful prognostic predictor in patients
with thyroid cancer. Clin Chem Lab Med. 2019;58:130-7. Crossref
33. Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C,
Fulco RA, et al. Risk-adapted management of differentiated
thyroid cancer assessed by a sensitive measurement of basal serum
thyroglobulin. J Clin Endocrinol Metab. 2011;96:1703-9. Crossref
34. Heemstra KA, Liu YY, Stokkel M, Kievit J, Corssmit E,
Pereira AM, et al. Serum thyroglobulin concentrations predict
disease-free remission and death in differentiated thyroid
carcinoma. Clin Endocrinol (Oxf). 2007;66:58-64.
35. Papaleontiou M, Haymart MR. New insights in risk stratification
of differentiated thyroid cancer. Curr Opin Oncol. 2014;26:1-7. Crossref
36. Glikson E, Alon E, Bedrin L, Talmi YP. Prognostic factors
in differentiated thyroid cancer revisited. Isr Med Assoc J.
2017;19:114-8.
| Attachment | Size |
|---|---|
| v24n1_Comparison.pdf | 619.04 KB |