Prognostic Impact of the Time Interval between Surgery and Postoperative Adjuvant Chemotherapy in Epithelial Carcinoma of the Ovary
ORIGINAL ARTICLE
Prognostic Impact of the Time Interval between Surgery and Postoperative Adjuvant Chemotherapy in Epithelial Carcinoma of
the Ovary
JNS Cheng, WWL Yip, B Chan, FCS Wong
Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr JNS Cheng, Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong. Email: cns325@ha.org.hk
Submitted: 20 Jul 2020; Accepted: 16 Nov 2020.
Contributors: All authors designed the study, acquired and analysed the data. JNSC and BC drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: The research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by the New Territories West Cluster Research Ethics Committee (Ref NTWC/REC/20084). The need
for patients to provide written consent was waived for this retrospective study.
Abstract
Introduction
Multiple studies have evaluated the prognostic impact of the time interval (TI) between initial surgery
and adjuvant chemotherapy for epithelial ovarian cancer with different time intervals and inconclusive results. The
aim of the present study was to evaluate the prognostic impact of a longer interval of 42 days.
Methods
In a retrospective single-centre analysis, data were collected for all patients with epithelial ovarian cancer
treated between 2007 and 2014. We divided patients by TI: ≤42 days and >42 days. The disease-free survival and
overall survival (OS) between the two groups were compared. A Cox regression model was used to evaluate different
prognostic factors. A p value <0.05 was considered statistically significant.
Results
The median follow-up time was 73 months. Among those with postoperative residual disease (n = 30), TI
of >42 days was associated with significantly worse OS (hazard ratio = 3.37, 95% confidence interval = 1.23-9.25,
p = 0.02). In cases with residual disease after surgery, the Cox proportional model showed the presence of ascites
(p = 0.03) and postoperative CA125 level (p = 0.03) were independent prognostic factors for DFS. TI >42 days
(p = 0.03) was an independent negative prognostic factor for OS along with grading (p = 0.05) and presence of
ascites (p < 0.01).
Conclusion
Our study showed that patients with residual disease after initial surgery had inferior OS when TI was
>42 days. Adjuvant chemotherapy in these patients should be started ≤42 days after surgery.
Key Words: Carcinoma, ovarian epithelial; Chemotherapy, adjuvant; Prognosis
中文摘要
上皮性卵巢癌的手術和術後輔助化療的時間間隔對預後的影響
鄭雁心、葉穎鈴、陳柏霖、黃志成
引言
多項研究已評估上皮性卵巢癌的手術與術後輔助化療的時間間隔對預後的影響,但使用的時
間間隔不同且結果尚無定論。本研究旨在評估較長的42天間隔的預後影響。
方法
在一項回顧性單中心分析中收集2007年至2014年間治療的所有上皮性卵巢癌患者的數據。我
們將患者分成兩組,包括手術與術後輔助化療的時間間隔(1)42天或以下以及(2)超過42天,並
比較兩組間的無病存活期和總存活期。使用Cox迴歸模型評估不同的預後因素。p值<0.05被認為具統
計學意義。
結果
中位隨訪時間為73個月。在具有術後殘留疾病(n = 30)的患者中,兩種治療的時間間隔超
過42天與整體存活率顯著下降相關(危險比 = 3.37,95%置信區間 = 1.23-9.25,p = 0.02)。對於術
後殘留疾病的患者,Cox迴歸模型顯示出現腹水(p = 0.03)和術後CA125水平(p = 0.03)是無病存
活期的獨立預後因素。兩種治療的時間間隔超過42天(p = 0.03)、疾病等級(p = 0.05)和出現腹水
(p < 0.01)是總存活的獨立陰性預後因素。
結論
我們的研究表明,當初始手術與輔助化療的時間間隔超過42天時,初次手術後有殘留疾病的
患者的總存活期較差。這些患者的輔助化療應在手術後42天或之前開始。
INTRODUCTION
Epithelial ovarian cancer is one of the most lethal
gynaecological cancers. According to the data released
by the Hong Kong Cancer Registry in 2019, ovarian
cancer was the 6th most common cancer and the 7th
leading cause of cancer-related deaths among women
in 2017.[1] The standard treatment for epithelial ovarian
cancer remains surgery with optimal debulking followed
by adjuvant chemotherapy.
Preclinical models had shown surgical removal of any
one of several tumours might accelerate the growth of
the residual tumours.[2] [3] Studies of cancers including
primary breast cancer, colorectal cancer, gastric cancer
and pancreatic cancer have reported significantly worse
outcomes with a delay in the initiation of systemic
therapy after surgery.[4] [5] [6] [7] [8]
Focusing on epithelial ovarian cancer, there is always a
struggle between earlier initiation of systemic therapy
after surgery and allowing more time for postoperative
recovery. Most patients with residual disease should
benefit from earlier chemotherapy after debulking
surgery. However, debulking surgery is a major operation that carries significant morbidity. Patients
usually require significant time for wound healing and
nutritional recovery. Surgical series reported a range
of inpatient stays of 4 to 14 days after primary surgical
staging for patients with ovarian cancer.[9] [10] Data from a
study focusing on primary surgery for ovarian cancer
versus neoadjuvant chemotherapy also reported a median
time to initiation of chemotherapy after primary surgery
in advanced ovarian cancer of 32 days, with a range of
5 to 82 days.[11]
Few studies have used a cut-off of 42 days. The study
by Paulsen et al[12] mainly focused on locally advanced
disease and it showed a significant negative impact on
overall survival (OS) if adjuvant chemotherapy was
delayed ≥6 weeks. Another study by Wright et al[13]
focused on patients aged >65 years with locally
advanced disease also found a significant negative
impact on OS if adjuvant chemotherapy was delayed
≥6 weeks. Focusing on early-stage disease, only one study
investigated the prognostic impact of administration of
chemotherapy <2, 2 to 4, or >4 weeks after surgery and
found no significant impact on disease-free survival
(DFS) or OS.[14] Thus, it was uncertain if a delay of >42 days after surgery would have any prognostic
impact on ovarian cancer.
The aim of this study was to evaluate the prognostic
impact of time interval (TI) in epithelial patients
with ovarian cancer in a single centre with a
standardised protocol on use of adjuvant chemotherapy
and a detailed record of residual disease after
surgery.
METHODS
Study Design
This retrospective study was conducted on the data of
all patients who underwent primary surgical staging
with debulking followed by at least one dose of adjuvant
chemotherapy at the Department of Clinical Oncology,
Tuen Mun Hospital between 1 January 2007 and 31
December 2014.
Inclusion Criteria
Cases of epithelial ovarian, fallopian tube, or primary
peritoneal carcinoma aged >18 years, undergoing
surgery with the intention of maximum debulking and
followed by at least one dose of adjuvant chemotherapy,
were included in this study. Cases receiving neoadjuvant
chemotherapy, with incomplete information on
chemotherapy administration or starting chemotherapy
>90 days after surgery were excluded.
Case Selection
The Clinical Data Analysis and Reporting System of
the Hospital Authority was used to identify all cases of
epithelial ovarian, fallopian tube or primary peritoneal
cancer first seen at the Department of Clinical Oncology
between 1 January 2007 and 31 December 2014 in Tuen
Mun Hospital. Only those cases fulfilling the inclusion
criteria were included.
EVALUATION OF OUTCOME
MEASURES
All patients were regularly followed up by an oncologist
after the completion of adjuvant chemotherapy. The
follow-up procedure in our department was every 3 to
4 months for the first to second year, every 4 to 6 months
for the third to fourth year, every 9 to 12 months for the
fifth year, and yearly afterwards. Clinical assessment
with history taking and physical examination would
be done on every follow-up. Serum CA125 test was
advised to be included at every visit. Imaging studies
were performed when there was clinical suspicion of
disease recurrence.
Patients’ demographic data, including date of birth and
date of death were collected from the patient medical
records.
The tumour stage and histological diagnosis of each
patient were determined according to the International
Federation of Gynaecology and Obstetrics criteria
and the histologic typing system of the World Health
Organization.[15] [16] Tumours were graded as either well
differentiated (Grade 1), moderately differentiated
(Grade 2), or poorly differentiated (Grade 3). Surgical
reports, inpatient discharge summaries, histology
reports, chemotherapy charts, and consultation notes of
individual cases were collected and reviewed.
The consultation notes of every follow-up were
reviewed. The progress of each case from the date of
surgery, including any recurrences and their dates, and
the date of death or latest follow-up date were recorded.
Outcome Measurements
The primary endpoint of this study was OS, which
was defined as the TI from surgery to a patient’s death.
The secondary endpoint was DFS, which was defined
as the TI from surgery to the date when the patient
was diagnosed with recurrence or the date of death,
whichever occurred first. The cut-off date for follow-up
was 17 July 2020. Imaging or histology was required
to diagnose recurrence; a rise in CA125 alone was
considered insufficient for diagnosis.
Sample Size and Rationale
Sample size calculation was estimated using the open
source software Power and Sample Size Program
version 3.0 for survival analysis with log rank tests.
On review of previous evidence, reported effect size
ranged from 1.02 to 3.44, assuming two tails and aiming
to detect an effect size of 1.7. Assuming achievement
of a significance level of 0.05, median survival for the
patients with TI >42 days of approximately 50 months,
and power = 0.7, the required sample size would be 134.
If the power were to be increased to 0.8, the sample
size required would be 170. However, as this study is a
single-centre retrospective study, including more cases
would involve gathering the data of cases who were first
seen by oncology before 2007; this was not possible as
records before that year had been destroyed.
Statistics
The TIs from surgery to chemotherapy of all included
cases were calculated. Patients were divided into two groups according to the TI of ≤42 days or >42 days.
The 42-day cut-off point was chosen because most
patients who received adjuvant chemotherapy >42 days
after initial surgery were excluded from most clinical
chemotherapy trial protocols.[11] [17] [18] Therefore, those
patients were among the least studied population.
SPSS (Window version 26.0; IBM Corp, Armonk [NY],
United States) was used for the statistical analyses.
Descriptive statistics included frequency and percentage
for categorical variables. Clinical data were compared
by Chi squared test or Fisher’s exact test for categorical
variables.
To visualise the crude DFS and OS for the two
groups with different TIs, Kaplan–Meier curves were
constructed. A log rank estimate was used to compare
the number of recurrences and deaths between the two
TI groups. The same analysis was also performed after
dividing patients into those with or without residual
disease. Cox regression analysis was further performed
to quantify the effect.
The Cox proportional hazards analysis was used to
evaluate the effect of different prognostic factors. A
p value of <0.05 was considered statistically significant
and all p values reported were two sided. Prognostic
factors included in the analysis were patient’s age at
operation, performance status, stage, histology, tumour
grading, size of residual, presence or absence of ascites,
number of chemotherapy cycles given, postoperative
CA125 value, and the TI between surgery and adjuvant
chemotherapy. The reason for choosing the above factors
was based on reviewing the significant prognostic factors
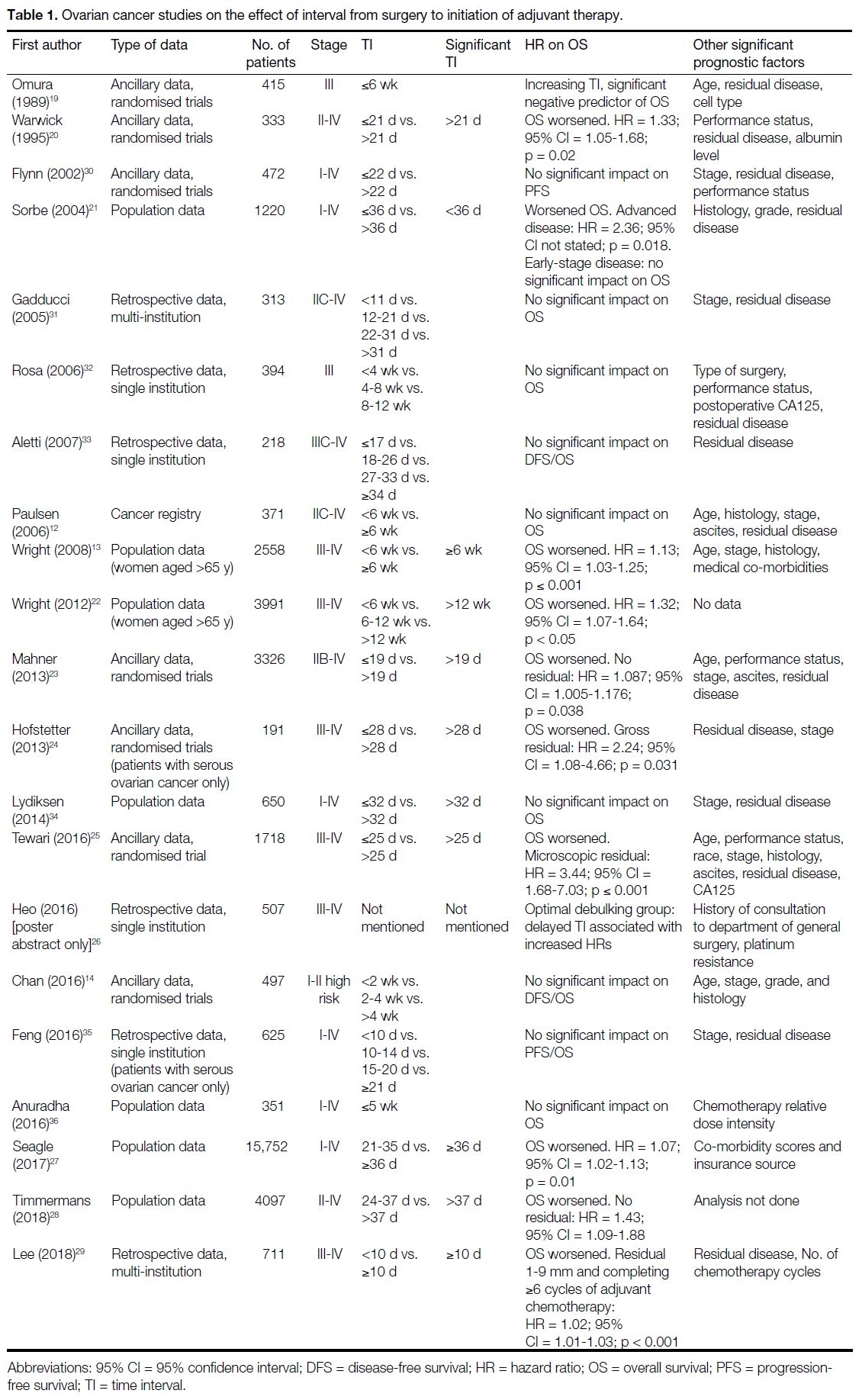
found in previous studies (Table 1 [12] [13] [14],[19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36]).
Table 1. Ovarian cancer studies on the effect of interval from surgery to initiation of adjuvant therapy.
Ethical Considerations
This study was approved by New Territories West
Cluster Research Ethics Committee (EC Ref. No.:
NTWC/REC/2084). The need for informed consent was
waived.
RESULTS
Characteristics of the Study Population
A total of 133 cases were included in the study. The
baseline characteristics of the study subjects are
summarised in Table 2. The median follow-up duration
was 72.6 (1.8-155.9) months. The median DFS and OS
were not reached. The most common disease stage in
the current population was stage IC (33.8%) and stage
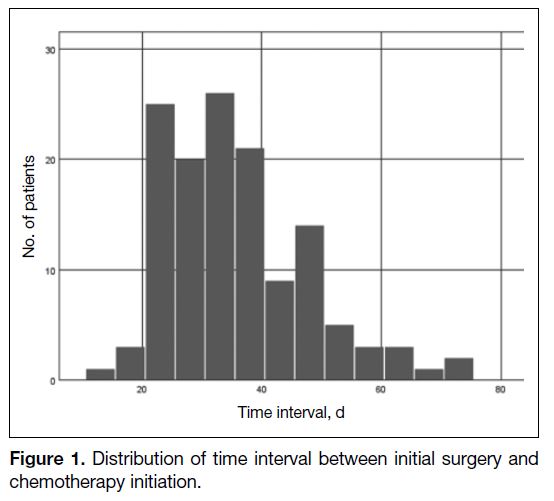
IIIC (24.8%). The median TI from surgery to adjuvant chemotherapy was 34 days (interquartile range, 27-42
days) [Figure 1]. In total, 98 patients had TI of ≤42 days and 35 patients had TI of >42 days.
Table 2. Characteristics of patients with regard to different
intervals from surgery to start of chemotherapy.
Figure 1. Distribution of time interval between initial surgery and chemotherapy initiation.
In all, 121 (91%) patients achieved optimal debulking
while only 12 (9%) patients had suboptimal debulking.
For those with optimal debulking, 18 had residual disease
of ≤1 cm. For those with suboptimal debulking, residual
disease ranged from 1.5 to 10 cm.
Patient characteristics across the two groups were
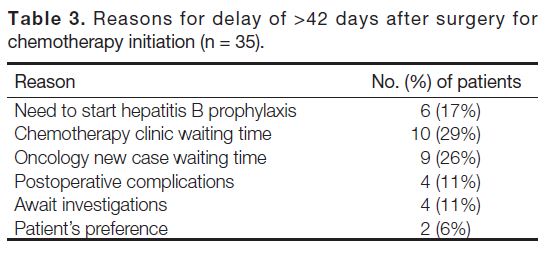
similar. Reasons for delaying initiation of chemotherapy
to >42 days after surgery are summarised in Table 3. In
all, 29% of the delays was due to chemotherapy clinic
waiting time while 26% were due to oncology new case
waiting time. There was also a delay in 17% of cases
due to the need to start hepatitis B prophylaxis before
chemotherapy, as hepatitis B carrier status is prevalent
in our area.
Table 3. Reasons for delay of >42 days after surgery for chemotherapy initiation (n = 35).
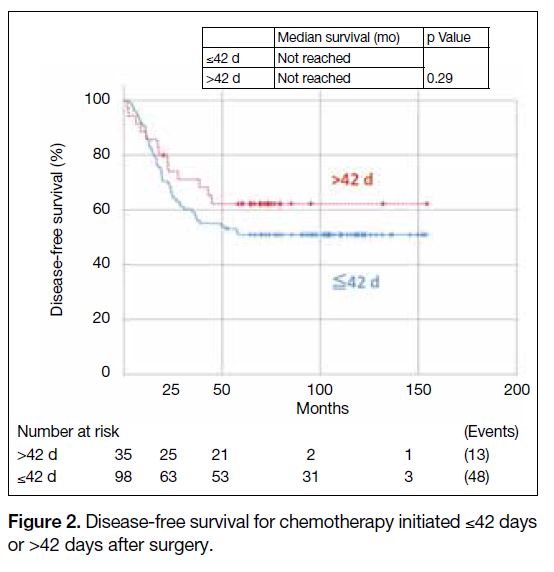
Disease-Free Survival
There was no significant effect on DFS when comparing
patients with TI of ≤42 days versus >42 days (hazard
ratio [HR] = 0.72, 95% confidence [CI] = 0.39-1.32,
p = 0.29) [Figure 2]. The 5-year DFS rate for patients
with TI of ≤42 days was 51.0% and that for patients with
TI of >42 days was 62.2%.
Figure 2. Disease-free survival for chemotherapy initiated ≤42 days or >42 days after surgery.
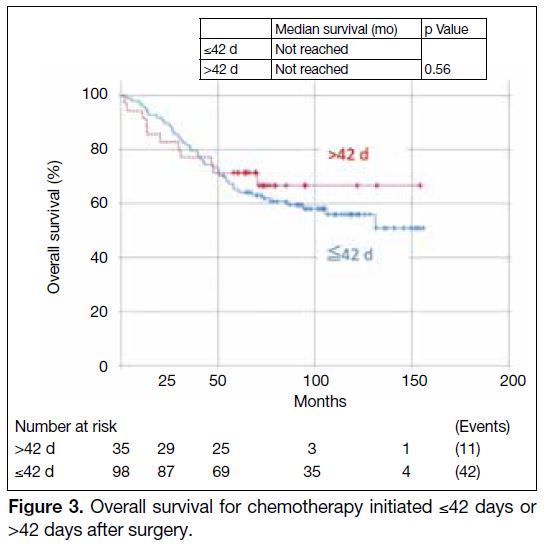
Overall Survival
There was no significant effect on OS when comparing
TIs of ≤42 days versus >42 days in all cases (HR = 0.82,
95% CI = 0.42-1.60, p = 0.56) [Figure 3]. The 5-year OS
rates for patients with TI of ≤42 days was 64.7% and that
for patients with TI of >42 days was 71.4%.
Figure 3. Overall survival for chemotherapy initiated ≤42 days or >42 days after surgery.
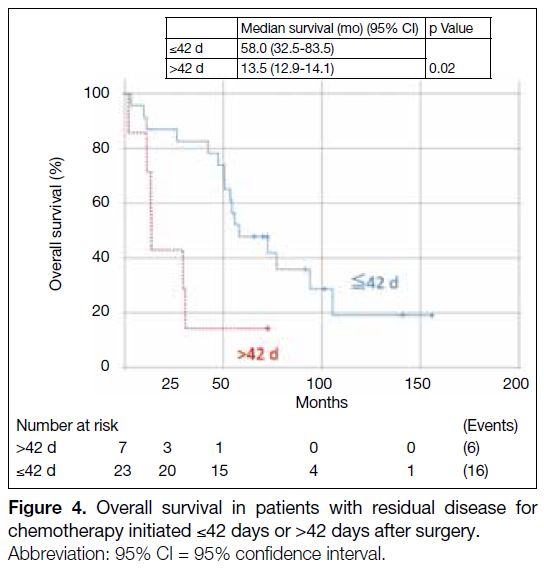
Subgroup Analysis with Presence or Absence
of Residual Disease
For patients with residual disease (n = 30), their OS was
statistically significantly worse for patients with TI of
>42 days (HR = 3.37, 95% CI = 1.23-9.25, p = 0.02)
[Figure 4]. The median OS for patients with TI of
≤42 days was 58 months (95% CI = 32.5-83.5) and that
for patients with TI >42 days was 13.5 months (95% CI =
12.9-14.1). The 5-year OS rate for patients with TI of
≤42 days was 65.2% and that for patients with TI of
>42 days was 14.3%.
Figure 4. Overall survival in patients with residual disease for chemotherapy initiated ≤42 days or >42 days after surgery.
There was no significant difference in DFS when
comparing patients with TI of ≤42 days versus >42 days
among patients with residual disease (HR = 1.77, 95%
CI = 0.71-4.48, p = 0.22).
For patients with no residual disease after surgery, there
was no DFS or OS difference when comparing patients
with TI of ≤42 days and >42 days (HR = 2.59, 95% CI =
0.26-1.35, p = 0.21 and HR = 0.50, 95% CI = 0.19-1.30,
p = 0.16, respectively).
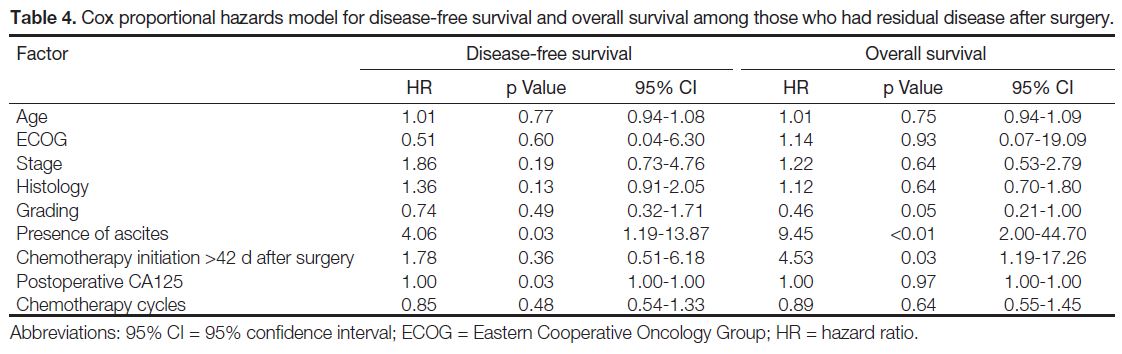
Prognostic Factors
In cases with residual disease after surgery, the Cox
proportional model showed the presence of ascites
(p = 0.03) and postoperative CA125 level (p = 0.03) were
independent prognostic factors for DFS. TI >42 days
(p = 0.03) was an independent negative prognostic factor
for OS (Table 4) along with grading (p = 0.05) and
presence of ascites (p < 0.01).
Table 4. Cox proportional hazards model for disease-free survival and overall survival among those who had residual disease after surgery.
DISCUSSION
The current study did not find an effect of TI on DFS
or OS in patients with epithelial ovarian cancer without
residual disease. However, for those with residual
disease, delaying chemotherapy to >42 days after surgery
was significantly associated with shorter OS.
It is well known that adjuvant chemotherapy is associated
with improved survival in epithelial ovarian cancer.[37]
However, patients do recur after surgery, especially those
with residual disease. In a study by Polterauer et al,[38] 3-year OS rates were 72.4%, 65.8%, and 45.2% for
patients with no residual disease, minimal residual
disease, and gross residual disease (>1 cm), respectively.
Our study showed there was a statistically significant
shortening of OS in patients with residual disease and a
TI of >42 days. We had 82 (61.7%) cases with stage I/II
disease and 51 (38.3%) cases with stage III/IV disease.
Our findings concur with a study by Seagle et al[27]
involving cases in stage I to IV that revealed a negative
prognostic effect of delaying chemotherapy ≥36 days
after surgery. That study consisted mainly of stage
III or IV patients (55.7% and 16.1%, respectively).
Tewari et al[25] reported in stage IV disease patients with
microscopic residual disease that a >25 days interval
from surgery to adjuvant chemotherapy was associated
with a worse OS. Lee et al[29] suggested that patients
with residual disease size ranging from 1 to 9 mm after
surgery were associated with significantly worsened
OS when there was delay in initiating chemotherapy of
≥10 days after surgery. Although their study was limited
to patients with serous ovarian cancer, Hofstetter et al[24]
suggested that there would be a worsened OS with
HRs of 2.24 for patients with gross residual disease
after primary surgery and chemotherapy initiated after
a TI >28 days. Our study also had similar findings.
However, one of the limitations in interpreting our
data is that our study sample size is small, with only
30 cases with residual disease after operation and only
seven initiating chemotherapy >42 days after surgery.
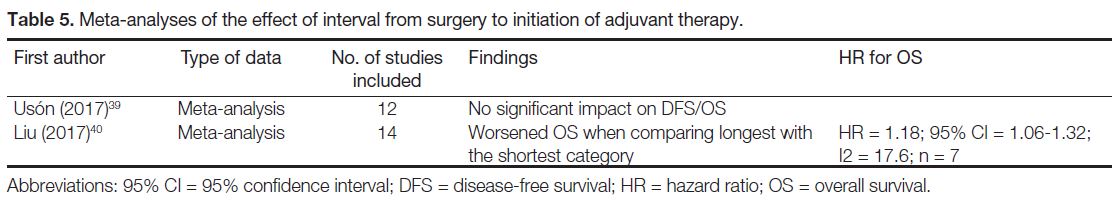
This finding can serve as hypothesis generation only
and further studies should be conducted to confirm this
hypothesis (Tables 1 [12] [13] [14],[19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] and 5 [39] [40]).
Table 5. Meta-analyses of the effect of interval from surgery to initiation of adjuvant therapy.
One of the greatest limitations in the current study
was sampling bias with confounding by indication. As
with many other retrospective studies, clinicians could
decide at their discretion on the timing of initiation of
chemotherapy. In our study, interestingly, we noted
a trend towards worse survival in those who initiated
chemotherapy earlier, although it was statistically
insignificant. It might be postulated that clinicians opted
to start chemotherapy early in patients who were deemed
to be at high risk of recurrence.
Another limitation of our study would be the follow-up
procedure. In our patients, the interval of surveillance
computed tomography (CT) or checking of tumour
marker CA125 was decided by the treating physician.
The lack of standardisation might have an impact on the
DFS. OS would be a more robust endpoint that is less
sensitive to the impact of diagnosing recurrence earlier
with more frequent imaging or blood tests. Indeed,
it had been shown that earlier initiation of palliative
chemotherapy based on elevated CA125 alone did not
improve OS.[41] [42]
The reasons for delaying initiation of chemotherapy in
this study were mainly the prolonged waiting time for
oncology new case appointments or chemotherapy clinic
appointments. Further arrangements of fast-track service
for this group of patients to improve their potential OS
should be considered. The prevalence of hepatitis B
carriage in the Asian population also warrants earlier detection of hepatitis B status to allow earlier initiation
of hepatitis B prophylaxis medications to avoid delays in
chemotherapy administration.
CONCLUSION
Our study showed that patients with residual disease after
initial surgery may have inferior OS when the adjuvant
chemotherapy is initiated >42 days after surgery. Further
studies should be conducted to see if this finding can be
reproduced. Adjuvant chemotherapy in these patients
should be started ≤42 days after surgery.
REFERENCES
1. Hospital Authority, Hong Kong SAR Government. Ovarian cancer
in 2017. Available from: https://www3.ha.org.hk/cancereg/pdf/factsheet/2017/ovary_2017.pdf. Accessed 5 Apr 2020.
2. Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the
growth and kinetics of residual tumor. Cancer Res. 1979;39:3861-5.
3. Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of
a growth-stimulating factor in serum following primary tumor
removal in mice. Cancer Res. 1989;49:1996-2001.
4. Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J,
et al. Impact on survival of time from definitive surgery to initiation
of adjuvant chemotherapy for early-stage breast cancer. J Clin
Oncol. 2006;24:4888-94. Crossref
5. Kupstas AR, Hoskin TL, Day CN, Habermann EB, Boughey JC.
Effect of surgery type on time to adjuvant chemotherapy and impact
of delay on breast cancer survival: a National Cancer Database
analysis. Ann Surg Oncol. 2019;26:3240-9. Crossref
6. Cai L, Tong Y, Zhu X, Shen K, Zhu J, Chen X. Prolonged time
to adjuvant chemotherapy initiation was associated with worse
disease outcome in triple negative breast cancer patients. Sci Rep.
2020;10:7029. Crossref
7. Petrelli F, Zaniboni A, Ghidini A, Ghidini M, Turati L, Pizzo C,
et al. Timing of adjuvant chemotherapy and survival in colorectal,
gastric, and pancreatic cancer. A systematic review and meta-analysis.
Cancers (Basel). 2019;11:550. Crossref
8. Noh GT, Han J, Cho MS, Hur H, Lee KY, Kim NK, et al. The
impact of early adjuvant chemotherapy in rectal cancer. PLoS One.
2020;15:e0228060. Crossref
9. Ghezzi F, Cromi A, Uccella S, Bergamini V, Tomera S, Franchi M,
et al. Laparoscopy versus laparotomy for the surgical management of
apparent early stage ovarian cancer. Gynecol Oncol. 2007;105:409-13. Crossref
10. Nezhat FR, DeNoble SM, Liu CS, Cho JE, Brown DN, Chuang L,
et al. The safety and efficacy of laparoscopic surgical staging and
debulking of apparent advanced stage ovarian, fallopian tube, and
primary peritoneal cancers. JSLS. 2010;14:155-68. Crossref
11. Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T,
et al. Primary chemotherapy versus primary surgery for newly
diagnosed advanced ovarian cancer (CHORUS): an open-label,
randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-57. Crossref
12. Paulsen T, Kaern J, Kjaerheim K, Haldorsen T, Tropé C. Influence
of interval between primary surgery and chemotherapy on short-term
survival of patients with advanced ovarian, tubal or peritoneal
cancer. Gynecol Oncol. 2006;102:447-52. Crossref
13. Wright JD, Doan T, McBride R, Jacobson J, Hershman D.
Variability in chemotherapy delivery for elderly women with
advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197-203. Crossref
14. Chan JK, Java JJ, Fuh K, Monk BJ, Kapp DS, Herzog T, et al. The
association between timing of initiation of adjuvant therapy and
the survival of early stage ovarian cancer patients — An analysis
of NRG Oncology/Gynecologic Oncology Group trials. Gynecol
Oncol. 2016;143:490-5. Crossref
15. Shepherd JH. Revised FIGO staging for gynaecological cancer. Br
J Obstet Gynaecol. 1989;96:889-92. Crossref
16. Scully R, Sobin LH, editors. Histological typing of ovarian tumours.
Heidelberg: Springer; 1999. Crossref
17. European Platform of Cancer Research. EORTC protocol 55981: a
randomized trial of Paclitaxel/Epirubicin/Carboplatin Combination
(TEC) versus Paclitaxel/Carboplatin(TC) in the treatment of women
with Advanced Ovarian Cancer. 1999. Available from: https://www.eortc.org/research_field/clinical-detail/55981/. Accessed 11
Apr 2020.
18. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T,
Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in
stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-53. Crossref
19. Omura GA, Bundy BN, Berek JS, Curry S, Delgado G, Mortel R.
Randomized trial of cyclophosphamide plus cisplatin with
or without doxorubicin in ovarian carcinoma: a Gynecologic
Oncology Group Study. J Clin Oncol. 1989;7:457-65. Crossref
20. Warwick J, Kehoe S, Earl H, Luesley D, Redman C, Chan KK.
Long-term follow-up of patients with advanced ovarian cancer
treated in randomised clinical trials. Br J Cancer. 1995;72:1513-7. Crossref
21. Sorbe B. Prognostic importance of the time interval from surgery
to chemotherapy in treatment of ovarian carcinoma. Int J Gynecol
Cancer. 2004;14:788-93. Crossref
22. Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN,
et al. Effect of radical cytoreductive surgery on omission and
delay of chemotherapy for advanced-stage ovarian cancer. Obstet
Gynecol. 2012;120:871-81. Crossref
23. Mahner S, Eulenburg C, Staehle A, Wegscheider K, Reuss A,
Pujade-Lauraine E, et al. Prognostic impact of the time interval
between surgery and chemotherapy in advanced ovarian cancer:
analysis of prospective randomised phase III trials. Eur J Cancer.
2013;49:142-9. Crossref
24. Hofstetter G, Concin N, Braicu I, Chekerov R, Sehouli J, Cadron I,
et al. The time interval from surgery to start of chemotherapy
significantly impacts prognosis in patients with advanced serous
ovarian carcinoma — analysis of patient data in the prospective
OVCAD study. Gynecol Oncol. 2013;131:15-20. Crossref
25. Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA. Early
initiation of chemotherapy following complete resection of
advanced ovarian cancer associated with improved survival:
NRG Oncology/Gynecologic Oncology Group study. Ann Oncol.
2016;27:114-21. Crossref
26. Heo EJ, Paik ES, Choi HJ, Kim WY, Lee YY, Choi CH, et al.
Impact of interval from definitive surgery to initiation of adjuvant
chemotherapy (ISC) on survival in advanced epithelial ovarian
cancer. Gynecologic Oncology. 2016 Jun 1;141:116-7. Crossref
27. Seagle BL, Butler SK, Strohl AE, Nieves-Neira W, Shahabi S.
Chemotherapy delay after primary debulking surgery for ovarian
cancer. Gynecol Oncol. 2017;144:260-5. Crossref
28. Timmermans M, van der Aa MA, Lalisang RI, Witteveen PO,
Van de Vijver KK, Kruitwagen RF, et al. Interval between
debulking surgery and adjuvant chemotherapy is associated with
overall survival in patients with advanced ovarian cancer. Gynecol
Oncol. 2018;150:446-50. Crossref
29. Lee YY, Lee JW, Lu L, Xu W, Kollara A, Brown T, et al. Impact
of interval from primary cytoreductive surgery to initiation of
adjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Gynecol Obstet. 2018;143:325-32. Crossref
30. Flynn PM, Paul J, Cruickshank DJ, Scottish Gynaecological
Cancer Trials Group. Does the interval from primary surgery
to chemotherapy influence progression-free survival in ovarian
cancer? Gynecol Oncol. 2002;86:354-7. Crossref
31. Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Maggioni A,
et al. Relationship between time interval from primary surgery to
the start of taxane-plus platinum-based chemotherapy and clinical
outcome of patients with advanced epithelial ovarian cancer:
results of a multicenter retrospective Italian study. J Clin Oncol.
2005;23:751-8. Crossref
32. Rosa DD, Clamp A, Mullamitha S, Ton NC, Lau S, Byrd L, et al.
The interval from surgery to chemotherapy in the treatment
of advanced epithelial ovarian carcinoma. Eur J Surg Oncol.
2006;32:588-91. Crossref
33. Aletti GD, Long HJ, Podratz KC, Cliby WA. Is time to chemotherapy a determinant of prognosis in advanced-stage ovarian
cancer? Gynecol Oncol. 2007;104:212-6. Crossref
34. Lydiksen L, Jensen-Fangel S, Blaakaer J. Is it possible to define an
optimal time for chemotherapy after surgery for ovarian cancer?
Gynecol Oncol. 2014;133:454-9. Crossref
35. Feng Z, Wen H, Bi R, Yang W, Wu X. Prognostic impact of the
time interval from primary surgery to intravenous chemotherapy in
high grade serous ovarian cancer. Gynecol Oncol. 2016;141:466-70. Crossref
36. Anuradha S, Donovan PJ, Webb PM, Brand AH, Goh J, Friedlander M, et al. Variations in adjuvant chemotherapy and survival in women with epithelial ovarian cancer — a population-based
study. Acta Oncol. 2016;55:226-33. Crossref
37. Kyrgiou M, Salanti G, Pavlidis N, Paraskevaidis E, Ioannidis JP.
Survival benefits with diverse chemotherapy regimens for ovarian
cancer: meta-analysis of multiple treatments. J Natl Cancer Inst.
2006;98:1655-63. Crossref
38. Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S,
et al. Prognostic value of residual tumor size in patients with
epithelial ovarian cancer FIGO stages IIA–IV: analysis of the
OVCAD data. Int J Gynecol Cancer. 2012;22:380-5. Crossref
39. Liu Y, Zhang T, Wu Q, Jiao Y, Gong T, Ma X, et al. Relationship
between initiation time of adjuvant chemotherapy and survival in
ovarian cancer patients: a dose-response meta-analysis of cohort
studies. Sci Rep. 2017;7:9461. Crossref
40. Usón PL Junior, Bugano DD, França MS, Antunes YP, Taranto P,
Kaliks RA, et al. Does time-to-chemotherapy impact the outcomes
of resected ovarian cancer? Meta-analysis of randomized and
observational data. Int J Gynecol Cancer. 2017;27:274-80. Crossref
41. Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A,
Jayson GC, et al. Early versus delayed treatment of relapsed ovarian
cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet.
2010;376:1155-63. Crossref
42. Rustin GJ, van der Burg ME. on behalf of MRC and EORTC
collaborators. A randomized trial in ovarian cancer (OC) of early
treatment of relapse based on CA125 level alone versus delayed
treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials). J Clin Oncol. 2009;27(Suppl 18):1. Crossref
| Attachment | Size |
|---|---|
| v24n1_Prognostic.pdf | 600.74 KB |