Imaging Findings and Clinical Perspectives of Acute Necrotising Encephalopathy: Report of Two Cases
CASE REPORT
Imaging Findings and Clinical Perspectives of Acute Necrotising Encephalopathy: Report of Two Cases
HL Wong, HY Lau, JCW Siu
Department of Radiology, Tuen Mun Hospital, Tuen Mun, Hong Kong
Correspondence: Dr HL Wong, Department of Radiology, Tuen Mun Hospital, Tuen Mun, Hong Kong. Email: holimw@gmail.com
Submitted: 2 Dec 2019; Accepted: 10 Dec 2019.
Contributors: JCWS designed the study. HLW acquired and analysed the data, and drafted the manuscript. JCWS and HYL critically revised the
manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed
consent for all treatments and procedures.
INTRODUCTION
First coined by Mizuguchi[1] in 1997, acute necrotising
encephalopathy (ANE) is a severe, uncommon type of
encephalopathy with distinctive imaging and clinical
features and a global distribution. Despite ongoing
studies, the exact pathogenesis of ANE is yet to be
determined and is still classified as an idiopathic disorder.
It is hypothesised that the disease is an immune response
to a prior, mostly viral, infection. With two illustrative
cases, we showcase the current understanding of the
pathogenesis, clinical and radiological presentation of
the disease as well as the latest treatment strategies.
CASE 1
A 2-year-old boy with good past health developed fever
and upper respiratory symptoms for 1 day. His symptoms
deteriorated overnight and he developed generalised
seizure. He was then urgently admitted to the Accident
and Emergency Department where the seizure was
aborted with anticonvulsant therapy. He was transferred
to the paediatric intensive care unit for further care.
A nasopharyngeal swab for rapid antigen test was
positive for influenza A. Despite empirical treatment
with vancomycin, rocephin, acyclovir, and oseltamivir,
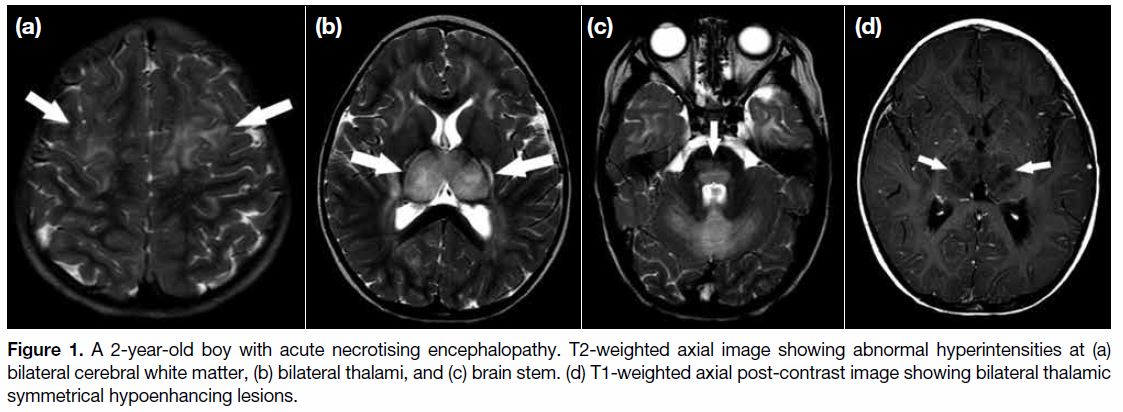
the patient developed breakthrough seizure. An urgent contrast magnetic resonance imaging (MRI) revealed
symmetrical T2 and fluid attenuated inversion recovery
hyperintensities with restricted diffusion over bilateral
thalami, white matter, and brain stem (Figure 1a-c).
Bilateral thalami were markedly swollen with central
hypo-enhancement (Figure 1d). Features were suggestive
of ANE.
Figure 1. A 2-year-old boy with acute necrotising encephalopathy. T2-weighted axial image showing abnormal hyperintensities at (a)
bilateral cerebral white matter, (b) bilateral thalami, and (c) brain stem. (d) T1-weighted axial post-contrast image showing bilateral thalamic
symmetrical hypoenhancing lesions.
The patient was started on intravenous pulse steroid
but his neurological condition deteriorated rapidly. A
follow-up computed tomography (CT) scan of the brain
showed diffuse cerebral oedema with effacement of
bilateral cerebral sulci and cisterns. Evidence of coning
was also observed. An urgent bilateral craniotomy and
decompression were performed. Despite the surgical
management, the patient’s condition remained poor.
Neurologically, the patient showed minimal brain
stem response and scored 4 (E1V1M2) in the Glasgow
Coma Scale. On day 5 after admission, the patient
succumbed.
CASE 2
A 6-year-old boy with good past health presented with
high fever, upper respiratory symptoms and transient
episodes of confusion. An urgent CT scan of the brain
performed on admission revealed no abnormality. A rapid antigen test on nasopharyngeal swab was positive
for influenza A.
The patient’s level of consciousness deteriorated rapidly:
Glasgow Coma Scale score dropped from 15 to 4 over 7
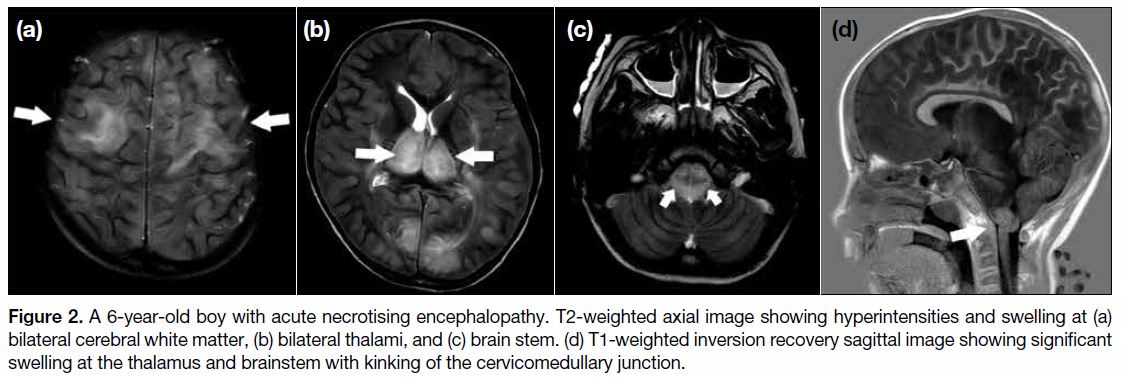
hours. MRI performed 12 hours after admission revealed
bilateral symmetrical T2 hyperintensities in the thalamus
and brain stem (Figure 2a and b). Associated bilateral
cerebral and cerebellar white matter hyperintensities
were also observed (Figure 2c). Features were suggestive
of ANE. There was significant swelling at the thalamus
and brain stem with kinking of the cervicomedullary
junction (Figure 2d). Crowding of the foramen magnum
was evident.
Figure 2. A 6-year-old boy with acute necrotising encephalopathy. T2-weighted axial image showing hyperintensities and swelling at (a)
bilateral cerebral white matter, (b) bilateral thalami, and (c) brain stem. (d) T1-weighted inversion recovery sagittal image showing significant
swelling at the thalamus and brainstem with kinking of the cervicomedullary junction.
A neurosurgical opinion was sought and the boy was
deemed too ill to benefit from surgery. He remained
comatose with no obvious return of any brainstem
function despite all medical support. The patient was
certified dead on day 12 after admission.
DISCUSSION
Pathogenesis and Aetiology of Acute
Necrotising Encephalopathy
The most widely accepted cause of ANE is
hypercytokinaemia.[2] Patients with ANE mount
an exaggerated immune response to infection by
releasing a high level of cytokines. There is evidence
that the level of various inflammatory mediators,
including different interleukins (IL-6, IL-15, IL-1β,
IL-10), tumour necrosis factor-α as well as interferon-γ,[2] [3] [4]
is raised in the serum as well as the cerebrospinal fluid.
This causes multiple system failure.[1]
Both environmental and host factors seem to play a role
in a predisposition to ANE.
A number of different infections are associated with
ANE and different pathogens have been reported,
including viruses and bacteria.[2] [5] Influenza virus is the
most common culprit.
There have been some recurrent cases reported within
certain families, suggesting a genetic predisposition.[5]
In recurrent cases, missense mutations in Ran-binding
protein 2 were identified as the susceptibility alleles.[5]
Clinical Presentation of Acute Necrotising
Encephalopathy
In general, the clinical presentation of ANE can be
classified into three stages: the prodromal stage, the
acute encephalopathy stage, and the recovery stage.
The presentation in the prodromal stage is highly
variable, due to the wide range of antecedent pathogens
possible. Symptoms may include fever, upper respiratory
symptoms, gastroenteritis, or skin rashes. Patients with
ANE may also present with more severe symptoms
including shock, disseminated intravascular coagulation
and even multiple organ failure.[5]
As the disease progresses, the acute encephalopathy
stage ensues during which neurological symptoms
will manifest rapidly. Seizures, an altered level of
consciousness and focal neurological deficits may
occur. The patient usually requires intensive care
support.
Although majority of the cases result in high morbidity
and mortality, milder forms with recovery have also
been reported.[5]
Radiological Findings and Imaging
Differential Diagnoses of Acute Necrotising
Encephalopathy
Contrary to the highly non-specific clinical presentations,
imaging features of ANE can usually help to narrow
down the differential diagnosis.[5] The list of differential
diagnoses is limited when rapid neurological deterioration
is taken into account.
The typical imaging presentation of ANE is multiple
symmetrical lesions with supra- and infra-tentorial brain
involvement. The thalami, brain stem, cerebral white
matter, and cerebellum[1] [2] are typical sites of involvement.
Spinal cord involvement is also occasionally reported.[5]
Among them, bilateral thalami involvement is the most
distinctive feature of ANE.[1]
It is also common for imaging features of ANE to change
over the clinical course.[1] [5] Significant oedema is seen
in the early phase. On CT, the lesions will show up as
symmetrical hypodense foci with mass effect. On MRI, the lesions will have a low T1 signal and high T2 signal.
There will also be fluid restriction on diffusion-weighted
imaging and apparent diffusion coefficient.[5]
As the disease progresses, imaging features change.
Oedema will subside and features of petechial
haemorrhage and necrosis may appear. On CT, this will
appear as new heterogeneous hyperdense foci within the
previously seen hypodensities.[1] [5] On MRI T1-weighted
image, there will be new hyperintensities within the
pre-existing hypointense lesions, while on T2-weighted
image they will show up as a hypointense centre
surrounded by a high signal rim.[5] Susceptibility weight
images and T2* gradient echo imaging are more able
to show the petechial haemorrhage that will appear as
low signal foci with blooming artefact.[5] The classically
described ANE imaging features of concentric structures,
tricolour pattern, or target-like appearance can be seen
on apparent diffusion coefficient images. The centre
of the lesion shows high signal with a hypointense rim
suggesting cytotoxic oedema. A further outer rim of
hyperintensity suggests vasogenic oedema.[5] [6]
With specific imaging features of ANE and a rapid
neurological decline, the imaging differential diagnoses
of ANE are limited. Entities that need to be considered
include acute disseminated encephalomyelitis, Reye’s
syndrome and Leigh’s syndrome. Acute disseminated
encephalomyelitis is associated with multiple bilateral
brain lesions bilaterally. Nonetheless the lesions do
not typically affect the brain symmetrically as in ANE.
Although Reye’s syndrome and Leigh’s syndrome may
present with symmetrical brain lesions, both entities are
characterised by the presence of lactic acidosis.
In both of our cases, the later imaging features were
not well demonstrated due to the rapid clinical course.
Unfortunately both patients succumbed before follow-up
imaging was available. However, the typical early
imaging features of ANE were well demonstrated.
Diagnostic Criteria of Acute Necrotising
Encephalopathy
Diagnosis of ANE is made if both clinical and imaging
findings are present and when similar-presenting
diseases have been excluded. Mizuguchi[1] have outlined
the diagnostic criteria (Table).
Table. Proposed diagnostic criteria for acute necrotising
encephalitis by Mizuguchi.[1]
Management and Prognosis of Acute
Necrotising Encephalopathy
There is no definitive recommended treatment for ANE. The mainstay of treatment is intensive care and
symptomatic treatment.
Theoretically, intravenous steroids, immunoglobulin,
and plasmapheresis should be effective in view of the
postulated immunological aetiology.[5] [7] High-dose
steroid is the most widely documented treatment.
Administration of steroids within 24 hours of symptom
onset is proposed to be related to an improved outcome.[5]
Therapeutic hypothermia has also been mentioned as a
supportive treatment.[5]
Despite treatment, ANE has a grave prognosis. The
mortality rate is reported to be about 30%. Complete
neurological recovery is observed in fewer than 10%
of patients and many survivors develop long-term
neurological sequelae.[1] [5]
CONCLUSION
Acute necrotising encephalitis is a severe neurological
complication of a common infection. The exact
pathogenesis is incompletely understood. The prognosis
of ANE is generally poor, although recent studies have
shown that starting treatment early may have a great
impact on the final outcome. Prompt diagnosis is vital to
ensure early treatment. The diagnosis of ANE is mainly
based on clinical and radiological features after exclusion
of other diseases with similar presentation.
The two cases we describe had typical imaging findings
and clinical presentation. One important limitation of our
case sharing is a lack of radio-pathological correlation.
In both patients, the cause of death was stated as ANE
and autopsy was waived in the absence of any other
suspected cause.
REFERENCES
1. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a
novel form of acute encephalopathy prevalent in Japan and Taiwan.
Brain Dev. 1997;19:81-92. Crossref
2. Munakata M, Kato R, Yokoyama H, Haginoya K, Tanaka Y,
Kayaba J, et al. Combined therapy with hypothermia and
anticytokine agents in influenza A encephalopathy. Brain Dev
2000;22:373-7. Crossref
3. Tabarki B, Thabet F, Al Shafi S, Al Adwani N, Chehab M,
Al Shahwan S. Acute necrotizing encephalopathy associated with
enterovirus infection. Brain Dev. 2013;35:454-7. Crossref
4. Vargas WS, Merchant S, Solomon G. Favorable outcomes in acute
necrotizing encephalopathy in a child treated with hypothermia.
Pediatr Neurol. 2012;46:387-9. Crossref
5. Wu X, Wu W, Pan W, Wu L, Liiu K, Zhang HL. Acute necrotizing
encephalopathy: an underrecognized clinicoradiologic disorder.
Mediators Inflam. 2015;2015:792578. Crossref
6. Ohsaka M, Houkin K, Takigami M, Koyanagi I. Acute necrotizing
encephalopathy associated with human herpesvirus-6 infection.
Pediatr Neurol. 2006;34:160-3. Crossref
7. Skelton BW, Hollingshead MC, Sledd AT, Philips CD, Castillo M.
Acute necrotizing encephalopathy of childhood: typical findings in
an atypical disease. Pediatr Radiol. 2008;38:810-3. Crossref
| Attachment | Size |
|---|---|
| v23n4_Imaging.pdf | 242.73 KB |