Pictorial Review of Paediatric Renal Transplant Vascular Complications
PICTORIAL ESSAY
Pictorial Review of Paediatric Renal Transplant Vascular
Complications
CWK Ng1, JTH Yeung2, KNY Pan1, WH Luk1, DCY Lui1, ALT Ma3, PC Tong3, THF Chan1
1 Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong
2 Department of Radiology, Yan Chai Hospital, Tsuen Wan, Hong Kong
3 Department of Paediatrics, Princess Margaret Hospital, Laichikok, Hong Kong
Correspondence: Dr CWK Ng, Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong. Email: carol26@gmail.com
Submitted: 24 Jul 2018; Accepted: 12 Oct 2018.
Contributors: All authors contributed to the concept and design of the study; CWKN acquired and analysed the data and drafted the manuscript;
all authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by the Hospital Authority Kowloon West Cluster Research Ethics Committee (Ref KW/EX-16-
048(97-07)).
INTRODUCTION
Renal transplantation offers the most effective long-term
renal replacement therapy for paediatric patients
with end-stage renal disease. It is associated with better
survival rates and quality of life than dialysis. Given
the technically demanding surgery with small-sized
vessels, paediatric patients are particularly susceptible to
vascular complications. They are an important cause of
morbidity after paediatric renal transplantation affecting
5% to 10% of patients.[1] Imaging plays an important role
in diagnosing these complications to facilitate timely
management. In this article, we will review the imaging
findings of paediatric renal transplant-related vascular
complications. The vascular complications can be
stratified into immediate (within a week), early (between
a week to a month), or late (>1 month) [Table].
Table. Timing of vascular complications after renal transplantation.
SURGICAL TECHNIQUE
The graft kidney is placed in the right or left iliac fossa.
The graft renal arteries and veins are anastomosed to the
ipsilateral common, external or internal iliac arteries and
veins.
RENAL ARTERY THROMBOSIS
Renal artery thrombosis is an uncommon yet devastating
major cause of early graft loss where flow in both the
main and the intrarenal arteries is absent.[2] One-third of
early graft loss is due to vascular thrombosis and occurs
in 3% to 4% of all paediatric renal transplants.[3] Typically
it is due to technical problems such as vessel kinking,
dissection, or torsion. Additional risk factors include
hypotension, multiple renal arteries, and unidentified
intimal flaps, young age of the recipient, young age of
the deceased donor, prolonged cold ischaemic time,
history of transplantation, and presence of acute tubular
necrosis.
Ultrasound is commonly used to diagnose renal infarcts.
Renal artery thrombosis is characterised by absent colour
flow and spectral Doppler waveforms within the main
renal vasculature, associated with absent parenchymal
perfusion on colour and power Doppler imaging
(Figure 1a, b). Although poor parenchymal perfusion
is also seen in hyperacute rejection and renal vein
thrombosis (RVT), in renal artery thrombosis a normal graft artery cannot be identified. An adjunct ultrasound
feature of renal artery thrombosis is a low resistive index
(RI) <0.6.[4] In cases where ultrasonographic identification
of the main renal artery is challenging due to technical
factors such as postoperative gas limiting the acoustic
window or a lack of operator experience, magnetic
resonance (MR) or contrast computed tomography (CT)
angiogram can provide a definitive diagnosis. The graft
artery will show absent flow signal on MR imaging or
a filling defect on contrast CT. Parenchymal perfusion
of the graft kidney on CT is also absent (Figure 1c, d).
Urgent thrombolysis or thrombectomy may occasionally salvage the kidney, but nephrectomy due to graft failure
is the more likely outcome.
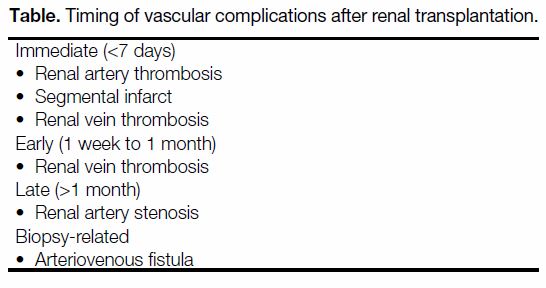
Figure 1. An 11-year-old girl with primary hyperoxaluria and end-stage renal failure complained of intense graft pain on day 10 after
surgery; blood test results revealed she was oliguric with deteriorating renal function. Her previous ultrasound scan on day 5 showed
normal renal vasculature and flow on colour Doppler. Ultrasound scan on day 10 showed no blood flow in the graft kidney on (a) colour
and (b) power Doppler studies. (c, d) Contrast-enhanced computed tomography showed no contrast enhancement in the graft kidney while
satisfactory contrast opacification of the inferior vena cava and aorta and bilateral iliac vessels were both seen (solid arrows). A concomitant
rim-like hyperdense, non-enhancing mass compatible with a perinephric haematoma is present (dashed arrows). Intraoperative findings
confirmed the presence of a perinephric haematoma and thrombosed graft kidney artery and vein with global infarction. The kidney was
non-salvageable and graft nephrectomy was performed.
SEGMENTAL INFARCT
Segmental infarcts are common in the early postoperative
period when the allograft has multiple renal arteries. It
is associated with an elevated lactate dehydrogenase
in blood tests although patients are often clinically
asymptomatic.[5]
On ultrasound, a segmental infarct has the appearance
of a wedge-shaped hypoechoic mass with poorly defined
margins (Figure 2a) or a hypoechoic mass with a well-defined
echogenic wall that shows absent Doppler flow
signal. Another cause of a similar grayscale appearance
is pyelonephritis, although colour Doppler will show
increased flow.
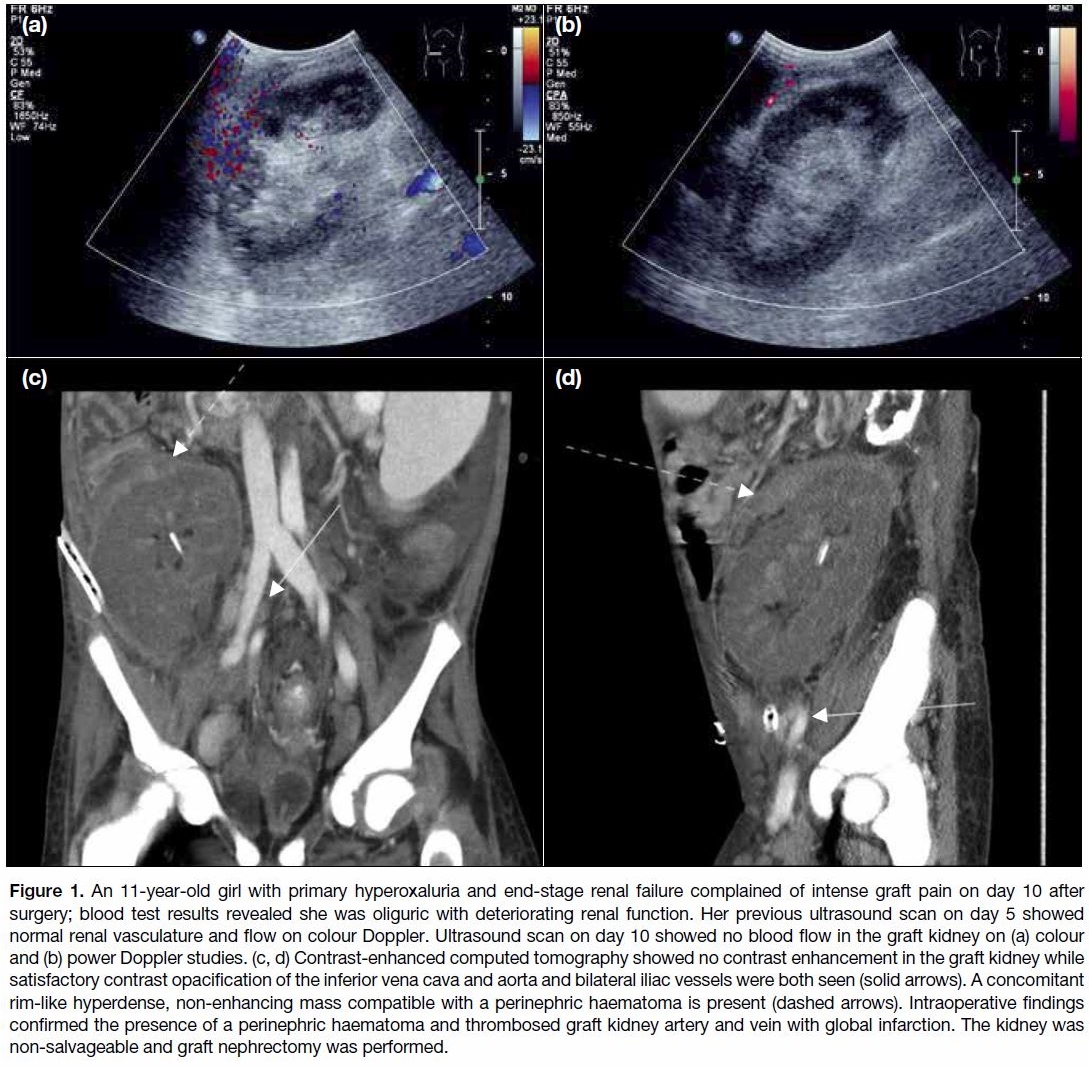
Figure 2. An 8-year-old girl with end-stage renal failure due to severe renal parenchymal disease underwent a cadaveric renal transplant.
The graft kidney had double renal arteries. The small upper pole renal artery was too small for anastomosis. (a) Power Doppler
ultrasound demonstrated a hypoechoic avascular wedge-shaped area in the upper pole of the graft kidney. (b) Technetium-labelled
mercaptoacetyltriglycine study demonstrated a focal wedge-shaped well-demarcated area of decreased perfusion and parenchymal
extraction (pink arrow) at the upper pole. Imaging findings were compatible with a focal segmental renal infarct in the upper pole.
Contrast-enhanced CT or scintigraphic study will
show wedge-shaped hypoenhancement or reduced
scintigraphic uptake at the segmental infarct (Figure 2b).[6]
Contrast-enhanced ultrasound is an alternative to CT in
diagnosing segmental infarct with no risk of contrast
nephropathy.[7] Most patients with segmental infarct can
be managed conservatively.
RENAL VEIN THROMBOSIS
Most cases of RVT occur within the first week of
transplantation. Nearly all cases are reported within the
first month. The incidence of RVT is 0.1% to 8.2%.[8] Risk
factors for developing thrombosis are donor age <6 or
>60 years, perioperative or postoperative haemodynamic instability, peritoneal dialysis, history of thrombosis,
cadaver organ, cold ischaemic time >24 hours, and
history of transplantation.[9] Patients usually present with
pain, graft swelling, and oliguria. Timely diagnosis of
RVT is crucial as there is a narrow therapeutic window
for graft salvage before irreversible ischaemia sets
in. Treatment includes prompt thrombolytic therapy,
transvascular mechanical thrombectomy or surgical
reoperation with thrombectomy. Beyond the therapeutic
window, the non-salvageable infarcted kidney will
require nephrectomy.
Ultrasound findings of RVT include diffuse swelling and
abnormal echogenicity in the graft parenchyma with an
elevated RI, main renal artery decreased peak systolic
velocity (PSV), and reversed diastolic flow.
Although acute rejection and acute tubular necrosis can
also present with a swollen kidney with elevated RI, a
specific sign on ultrasound for RVT is the absence of
venous flow on colour and spectral Doppler (Figure 3a).
On greyscale ultrasound, a thrombosed renal vein is seen
as a tubular structure in the renal hilum.
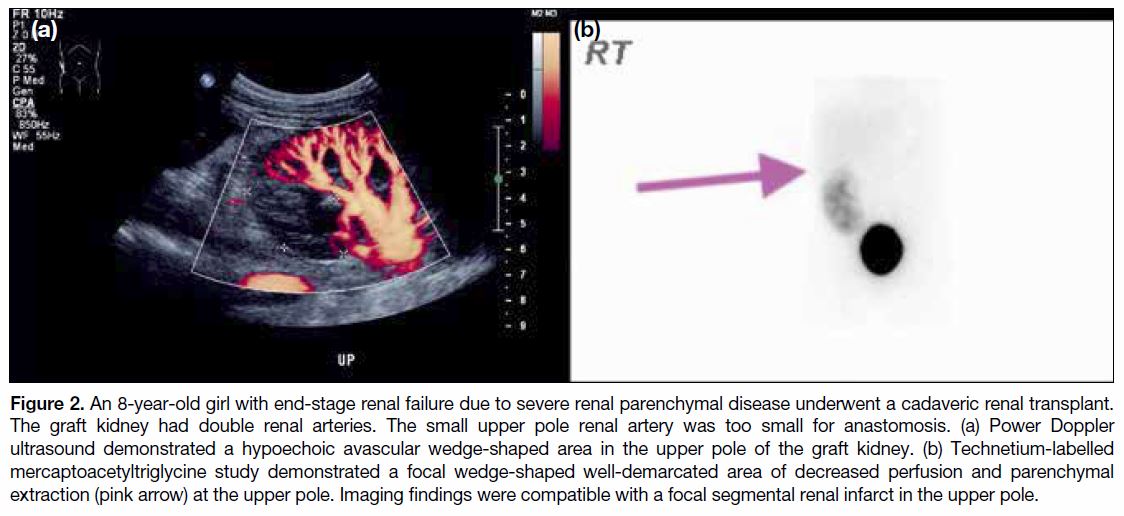
Figure 3. A 15-year-old boy with Noonan syndrome, cystic
dysplastic kidney, and end-stage renal failure underwent a
cadaveric renal transplant. Creatinine was persistently elevated.
Ultrasound showed markedly reduced graft kidney perfusion.
Renal vein colour flow was absent. (a) A thrombosed renal vein
was seen as a hypoechoic tubular structure in the renal hilum.
(b, c) Contrast computed tomography showed a hyperdense
non-enhancing, distended renal vein (white arrows) compatible
with renal vein thrombosis. The graft kidney showed markedly
reduced perfusion. Intraoperative findings confirmed renal vein
thrombosis, the graft kidney was severely congested with multiple
superficial ruptures. The graft could not be salvaged and graft
nephrectomy was performed.
RI is used as an adjunct to colour Doppler ultrasound
in graft kidneys. It is calculated using the following
formula: (PSV − end diastolic velocity) / PSV. There is a
variation of the upper limit of normal RI in the literature,
ranging from 0.7 to 0.8.[10] RI is used as a marker of
microcirculation injury and a sequela of interstitial
oedema in all forms of graft dysfunction, and is non-specifically
elevated in a range of conditions.[1] Elevated RI is correlated with early allograft dysfunction, but does
not show a correlation with long-term allograft survival.[11]
RVT on scintigraphy is revealed as decreased perfusion
and delayed cortical uptake, with prolonged cortical
retention and reduced excretion. A similar pattern can be
seen in parenchymal pathologies such as acute rejection
or pseudorejection.
If in doubt, CT with contrast (Figure 3b, c) can be
employed but further deterioration of renal function
is a concern for patients with raised creatinine. MR
venography can help confirm this complication but is not
as readily available as CT.
RENAL ARTERY STENOSIS
The incidence of transplant-related arterial stenosis
(TRAS) in paediatric patients ranges from 5% to 9%.[8]
Prolonged cold ischaemia time, occurrence of delayed
graft function, and cytomegalovirus infection are
recognised risk factors.[12] [13] An adjunctive risk factor
in young patients may be the diameter of the small
vessels and mismatch of anastomosed vessel size
due to the disproportional body weight of donor and
recipient.[14] Patients usually present clinically with
new-onset hypertension or worsening of pre-transplant
hypertension. Patients with TRAS often require high
dosages of antihypertensives for blood pressure control.
A small portion of TRAS can be clinically silent so
regular screening with Doppler ultrasound is essential.
Ultrasound is free of radiation and easily available, but
CT and MR angiograms can more accurately detect
the extent and site of arterial stenosis. Ultrasound will
show focal areas of colour aliasing due to increased flow
velocity. The ultrasound criteria for diagnosis of TRAS
in paediatric patients are based on studies in adults
since no paediatric-specific data have been published.
A wide range of PSV thresholds have been published
for detection of haemodynamically significant (>50%)
transplant-related renal artery stenosis (RAS) with a
range of sensitivities and specificities. Practically, a
threshold of 200 cm/s is often used with a sensitivity 90%
to 100% and specificity of 67% to 88%[1] (Figure 4a).
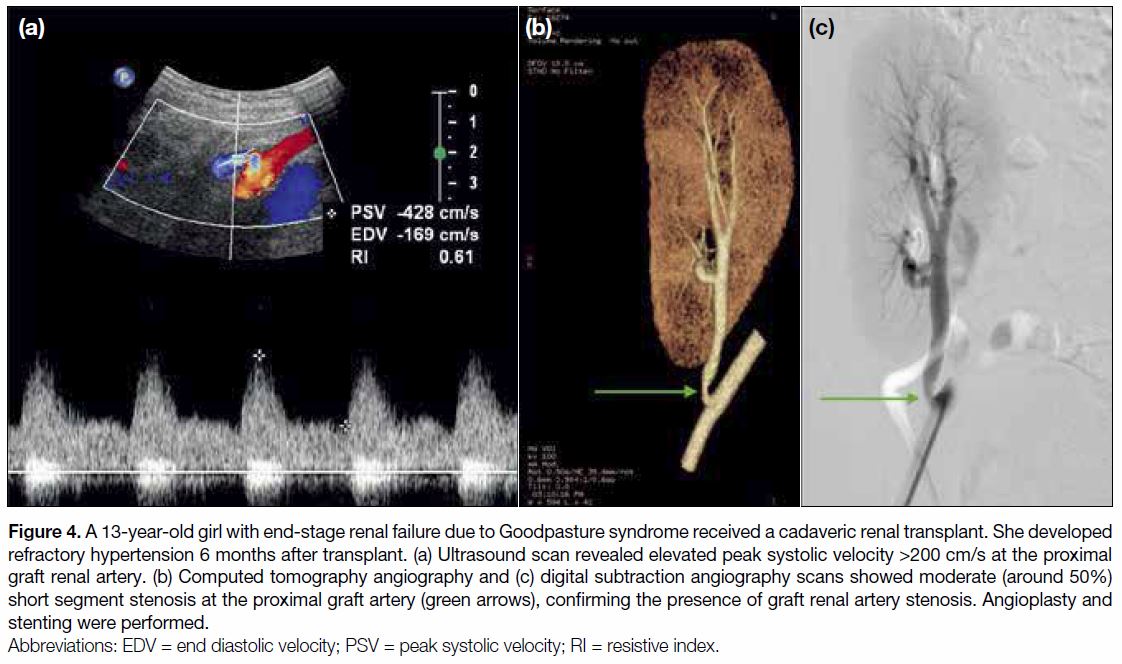
Figure 4. A 13-year-old girl with end-stage renal failure due to Goodpasture syndrome received a cadaveric renal transplant. She developed
refractory hypertension 6 months after transplant. (a) Ultrasound scan revealed elevated peak systolic velocity >200 cm/s at the proximal
graft renal artery. (b) Computed tomography angiography and (c) digital subtraction angiography scans showed moderate (around 50%)
short segment stenosis at the proximal graft artery (green arrows), confirming the presence of graft renal artery stenosis. Angioplasty and
stenting were performed.
To mitigate variation in PSV due to the difference
in systemic blood flow velocities, another Doppler
parameter used is the post-stenosis PSV: pre-stenosis
PSV ratio, that is, renal-to-iliac artery PSV, normally
<1.8 to 2.0.[15] This is again based on adult studies as
no relevant paediatric data have been published. The intra-arterial waveform of tardus-parvus pattern with a
slow rise in velocity is another sign of RAS.
Catheter-directed angiogram remains the gold standard
for evaluation of transplant renal artery stenosis
(Figure 4c). MR angiogram involves no radiation but is
less readily available than CT angiogram (Figure 4b).
Additional sedation may be required for MR compared
with CT due to the longer scan time. Renal scintigraphy
after angiotensin-converting enzyme inhibition has
fallen out of favour for diagnosing RAS owing to its low
sensitivity and high radiation exposure.
The mainstay of treatment for TRAS is percutaneous
angioplasty. Surgery is reserved for failed percutaneous
angioplasty with or without stenting and for anastomotic
stenoses.[14]
ARTERIOVENOUS FISTULA
A percutaneous renal biopsy is invaluable for surveillance
and for histologically diagnosing the aetiology of graft
dysfunction. Arteriovenous fistula (AVF) after biopsy
is more common in graft than native kidneys with an
incidence of 15% to 16%.[16] AVF after biopsy has an
incidence of around 7% in transplanted kidneys, of
which 0.3% to 0.4% are symptomatic. Risk factors for
AVF development include renal medullary disease, nephrocalcinosis, hypertension, renal insufficiency,
increased number of attempts, and depth of the biopsy.
80% to 95% of them resolve spontaneously without
treatment in 2 to 31 months.[17]
Small AVFs constitute the majority of cases and most
close spontaneously. Larger AVFs are less common
and can be symptomatic. Treatment is required for
symptomatic cases with haematuria, high-output cardiac
failure, vascular steal, and hypertension. Transarterial
embolisation is the treatment of choice. It is safe and
effective, with minimal loss of renal parenchyma. Longterm
graft survival is not affected by embolisation. Late
recurrence rate is low after treatment.[18]
The ultrasound appearance of AVFs is colour aliasing in
a circumscribed area of the renal parenchyma showing
turbulent flow with high-velocity arterial flow with a
reduced systolic-diastolic difference and high arterialised
venous flow on spectral analysis (Figure 5).
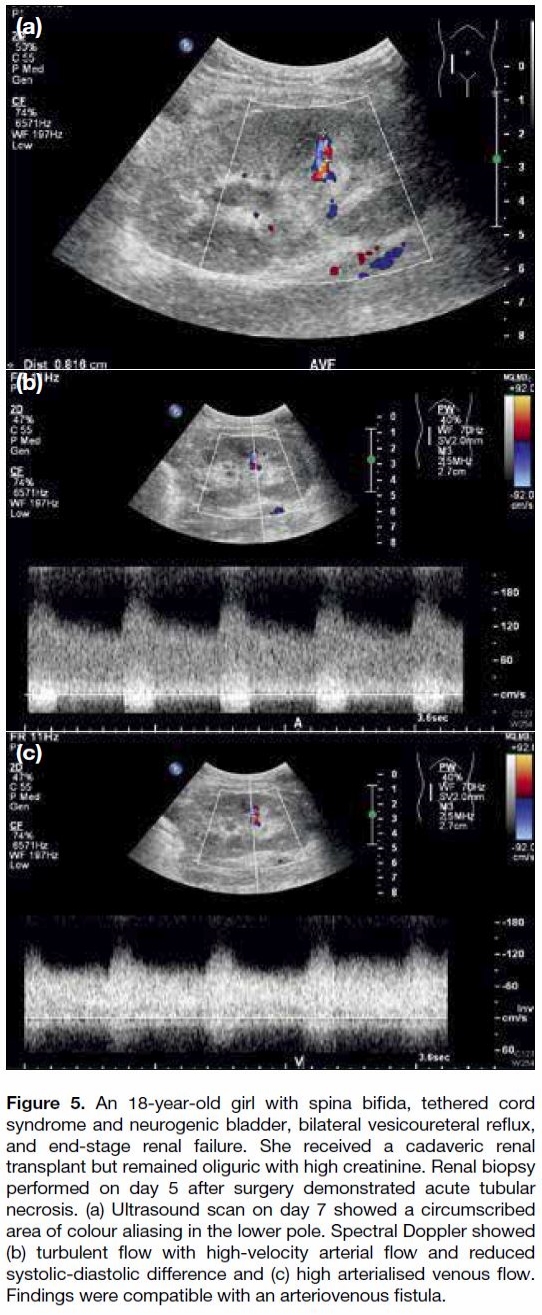
Figure 5. An 18-year-old girl with spina bifida, tethered cord
syndrome and neurogenic bladder, bilateral vesicoureteral reflux,
and end-stage renal failure. She received a cadaveric renal
transplant but remained oliguric with high creatinine. Renal biopsy
performed on day 5 after surgery demonstrated acute tubular
necrosis. (a) Ultrasound scan on day 7 showed a circumscribed
area of colour aliasing in the lower pole. Spectral Doppler showed
(b) turbulent flow with high-velocity arterial flow and reduced
systolic-diastolic difference and (c) high arterialised venous flow.
Findings were compatible with an arteriovenous fistula.
CONCLUSION
Vascular complications can range from those with
poor clinical outcomes such as renal artery thrombosis
or RVT, to less serious complications such as AVF or
segmental infarct. Renal artery thrombosis typically
occurs in the period immediately after the transplant.
REFERENCES
1. Nixon JN, Biyyam DR, Stanescu L, Philips GS, Finn LS, Parisi MT.
Imaging of pediatric renal transplants and their complications: a
pictorial review. Radiographics. 2013;33:1227-51. Crossref
2. Humar A, Matas AJ. Surgical complications after kidney
transplantation. Semin Dial. 2005;18:505-10. Crossref
3. Keller AK, Jorgensen TM, Jespersen B. Identification of risk factors
for vascular thrombosis may reduce early renal graft loss: a review
of recent literature. J Transplant. 2012;2012:793461. Crossref
4. Fananapazir G, Tse G, Corwin MT, Santhanakrishnan C, Perez RV,
McGahan JP, et al. Pediatric en bloc kidney transplants: clinical
and immediate postoperative us factors associated with vascular
thrombosis. Radiology. 2016;279:935-42. Crossref
5. Kanchanabat B, Siddins M, Coates T, Tie M, Russell CH, Mathew T,
et al. Segmental infarction with graft dysfunction: an emerging
syndrome in renal transplantation? Nephrol Dial Transplant.
2002;17:123-8. Crossref
6. McArthur C, Baxter GM: Current and potential renal applications
of contrast-enhanced ultrasound. Clin Radiol. 2012;67:909-22. Crossref
7. Bertolotto M, Martegani A, Aiani L, Zappetti R, Cernic S, Cova MA.
Value of contrast-enhanced ultrasonography for detecting renal
infarcts proven by contrast enhanced CT. A feasibility study. Eur
Radiol. 2008;18:376-83. Crossref
8. Ghirardo G, De Franceschi M, Vidal E, Vidoni A, Ramondo G,
Benetti E, et al. Transplant renal artery stenosis in children:
risk factors and outcome after endovascular treatment. Pediatric
Nephrol. 2014;29:461-7. Crossref
9. Singh A, Stablein D, Tejani A. Risk factors for vascular
thrombosis in pediatric renal transplantation: a special report of
the North American Pediatric Renal Transplant Cooperative Study.
Transplantation. 1997;63:1263-7. Crossref
10. Gholami S, Sarwal MM, Naesens M, Ringertz HG, Barth RA,
Balise RR, et al. Standardizing resistive indices in healthy pediatric
transplant recipients of adult-sized kidneys. Pediatr Transplant.
2010;14:126-31. Crossref
11. Melek E, Baskın E, Gulleroglu K, Uslu N, Kırnap M, Moray G,
et al. The predictive value of resistive index obtained by Doppler
ultrasonography early after renal transplantation on long-term
allograft function. Pediatr Transplant. 2017;21:10.1111/petr.12860. Crossref
12. Audard V, Matignon M, Hemery F, Snanoudj R, Desgranges P,
Anglade MC, et al. Risk factors and long-term outcome of transplant
artery stenosis in adult recipients after treatment by percutaneous
transluminal angioplasty. Am J Transplant. 2006;6:95-9. Crossref
13. Patel NH, Jindal RM, Wilkin T, Rose S, Johnson MS, Shah H, et al.
Renal arterial stenosis in renal allografts: retrospective study of
predisposing factors and outcome after percutaneous transluminal
angioplasty. Radiology. 2001;219:663-7. Crossref
14. Fontaine E, Bathelemy Y, Gagnadoux MF, Cukier J, Broyer M,
Beurton D. A review of 72 renal artery stenoses in a series of
715 kidney transplantations in children [in French]. Prog Urol.
1994;4:193-205.
15. de Morais RH, Muglia VF, Mamere AE, Garcia Pisi T, Saber LT,
Muglia VA, et al. Duplex Doppler sonography of transplant renal
artery stenosis. J Clin Ultrasound. 2003;31:135-41. Crossref
16. Shaheen F, Hakeem A, Singh M, Gojwari T, Shafi H, Wani M, et al.
Color Doppler findings of post-biopsy arteriovenous fistula in renal
transplant. Indian J Nephrol. 2008;18:123-3. Crossref
17. Omoloja AA, Racadio JM, McEnery PT. Post-biopsy renal
arteriovenous fistula. Pediatr Transplant. 2002;6:82-5. Crossref
18. Loffory R, Guiu B, Lambert A, Mousson C, Tanter Y, Martin L,
et al. Management of post-biopsy renal allograft arteriovenous
fistulas with selective arterial embolization: immediate and longterm
outcomes. Clin Radiol. 2008;63:657-65. Crossref