Fever of Unknown Origin with Fluorodeoxyglucose-crowned Dens Syndrome on Positron Emission Tomography: Case Reports
CASE REPORT
Fever of Unknown Origin with Fluorodeoxyglucose-crowned Dens Syndrome on Positron Emission Tomography: Case Reports
Y Yip, SC Wong, FPT Choi
Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Correspondence: Dr Y Yip, Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong. Email: jyipyu@gmail.com
Submitted: 24 Oct 2019; Accepted: 30 Oct 2019.
Contributors: YY and SCW designed the study. YY was responsible for acquisition of data. YY and FPTC analysed the data. YY wrote the
manuscript. All authors made critical revisions of the intellectual content of this manuscript. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This case report was approved by Hong Kong East Cluster Research Ethics Committee (Ref. HKECREC-2019-089). Informed
consent for publication was obtained from patients or their next of kin.
INTRODUCTION
The term crowned dens syndrome (CDS) was coined
four decades ago to first describe acute neck pain with
calcium crystal depositions that showed a crown-like
density surrounding the odontoid process (dens) on
frontal-view radiographs.[1] CDS remains an uncommon
condition that is often misdiagnosed as other infective,
inflammatory, or neoplastic disorders.[2] It has been
increasingly recognised as a peculiar manifestation of
calcium pyrophosphate deposition (CPPD) disease in
the aged population.[3] We describe two patients with
CDS and an unusual constellation of clinicoradiological
features who were investigated for fever of unknown
origin (FUO) by both gallium-67 scintigraphy and
fluorine-18 fluorodeoxyglucose (FDG) positron emission
tomography–computed tomography (PET-CT). Only the
latter revealed the diagnosis based on a rarely reported
imaging pattern.
CASE 1
An 82-year-old woman was admitted for biliary sepsis
complicated by hospital-acquired pneumonia. She
underwent endoscopic intervention and was prescribed
broad-spectrum antibiotics. Recurrent intermittent
fever persisted for 4 weeks with raised leucocyte count (17×109/L), erythrocyte sedimentation rate (>100 mm/h),
and C-reactive protein level (130 mg/L). The patient had
no localising symptoms. Gallium-67 scintigraphy for
FUO reported resolving pneumonia with mild activity
but could not localise other sources of infection. Her
fever persisted for another 4 weeks. Torso FDG PET-CT
was then performed. PET revealed polyarticular
FDG hyperactivity (maximum standardised uptake
values [SUVmax] 3.3-6.3) involving the shoulder,
sternocostoclavicular, wrist, lumbar apophyseal and
sacroiliac joints, and a characteristic pattern (SUVmax
4.0) around the dens of axis (Figure 1). CT detected subtle,
mottled high-attenuation deposits in retro-odontoid
soft tissues, and more deposits in the shoulder and
sternoclavicular articulations, and in the pubic symphysis
where it had no hyperactivity. Gallium-67 images were
reviewed and considered to show no abnormal uptake in
the cervical spine (Figure 2). The patient was diagnosed
as a probable case of CPPD disease solely based on
FDG PET-CT. She was prescribed colchicine and
low-dose prednisolone with rapid resolution of pyrexia
and inflammatory markers.
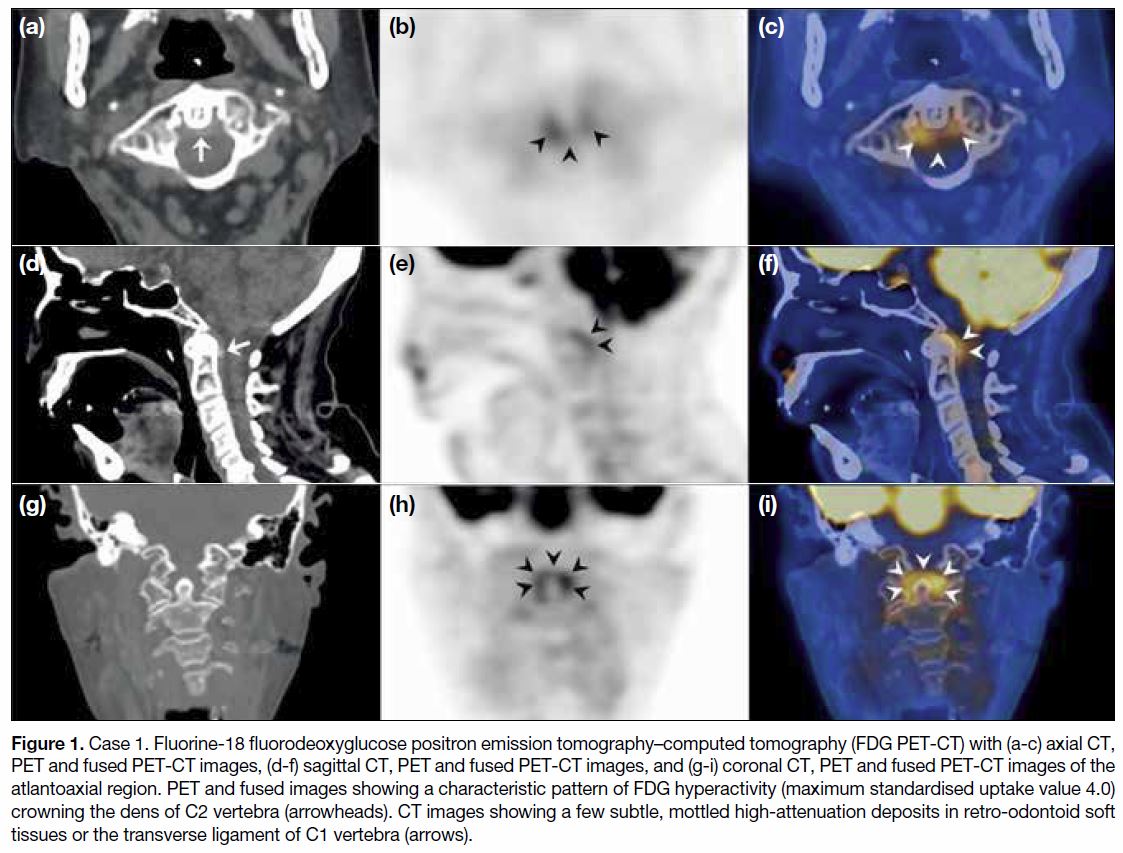
Figure 1. Case 1. Fluorine-18 fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET-CT) with (a-c) axial CT,
PET and fused PET-CT images, (d-f) sagittal CT, PET and fused PET-CT images, and (g-i) coronal CT, PET and fused PET-CT images of the
atlantoaxial region. PET and fused images showing a characteristic pattern of FDG hyperactivity (maximum standardised uptake value 4.0)
crowning the dens of C2 vertebra (arrowheads). CT images showing a few subtle, mottled high-attenuation deposits in retro-odontoid soft
tissues or the transverse ligament of C1 vertebra (arrows).
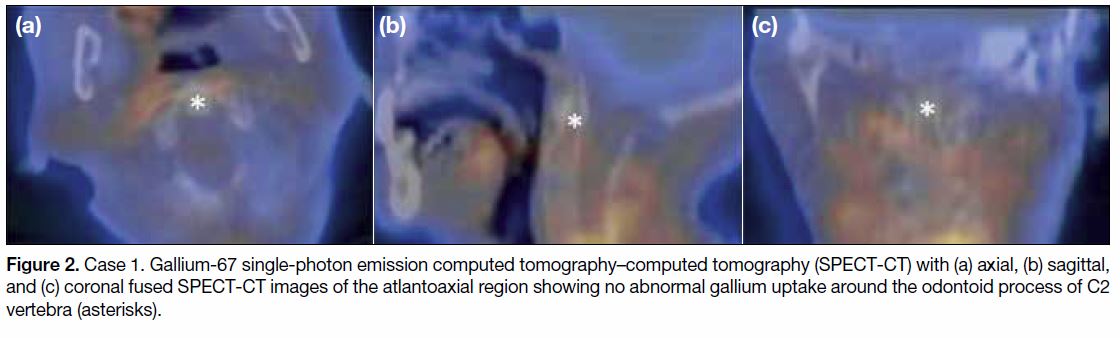
Figure 2. Case 1. Gallium-67 single-photon emission computed tomography–computed tomography (SPECT-CT) with (a) axial, (b) sagittal,
and (c) coronal fused SPECT-CT images of the atlantoaxial region showing no abnormal gallium uptake around the odontoid process of C2
vertebra (asterisks).
CASE 2
A female nonagenarian had a history of CPPD disease proven by knee synovial fluid analysis. She had repeat
admissions for pneumonia and septicaemia. Her
episodes evolved into FUO despite extensive workup
and prolonged courses of antibiotics. Gallium-67
scintigraphy demonstrated polyarticular uptake,
especially at the shoulders and knees. Cervical spine
had no abnormal gallium uptake. She underwent
image-guided shoulder arthrocentesis that yielded negative cultures and no crystals. Her fever persisted
on and off for another 6 weeks. FDG PET-CT was
then performed and confirmed polyarthropathy with
hyperactivity (SUVmax 2.8-4.8) over the shoulders,
sternoclavicular junctions, pubic symphysis, knees, and
with a characteristic pattern (SUVmax 3.7) around the
dens (Figure 3). She was prescribed colchicine and made
a rapid recovery.
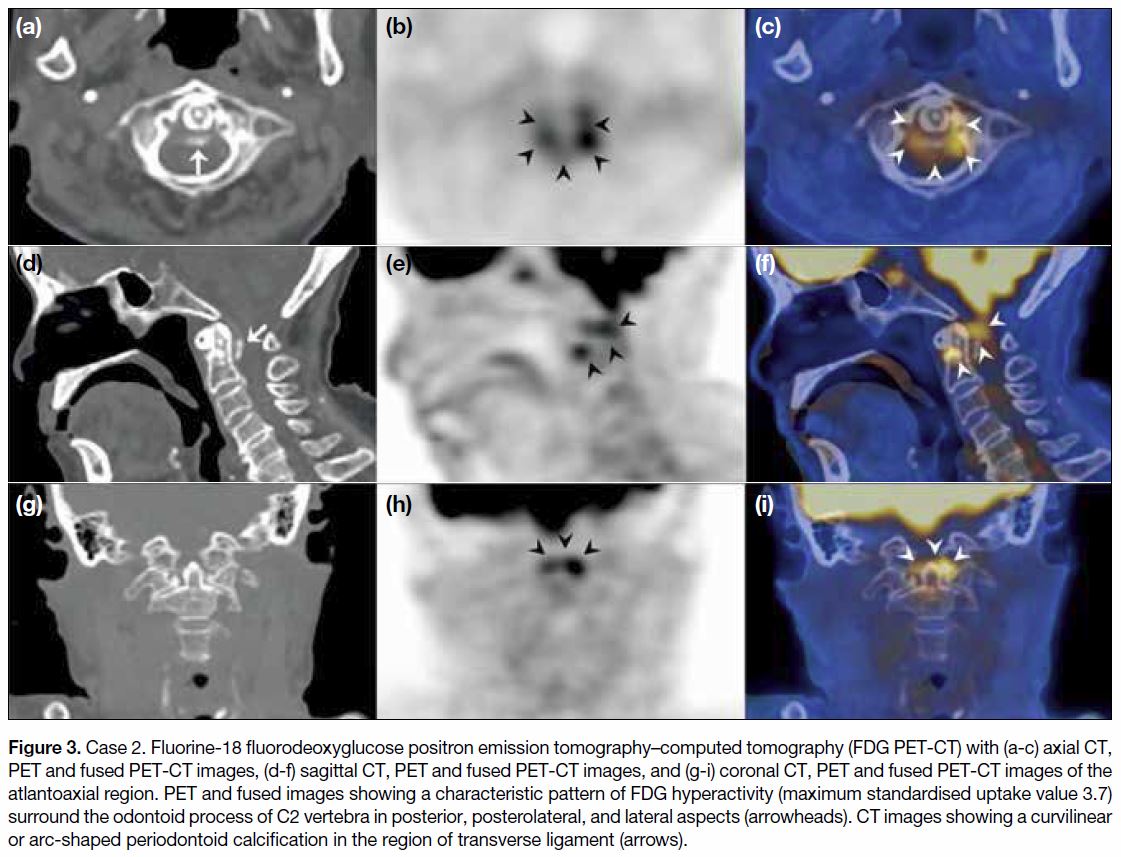
Figure 3. Case 2. Fluorine-18 fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET-CT) with (a-c) axial CT,
PET and fused PET-CT images, (d-f) sagittal CT, PET and fused PET-CT images, and (g-i) coronal CT, PET and fused PET-CT images of the
atlantoaxial region. PET and fused images showing a characteristic pattern of FDG hyperactivity (maximum standardised uptake value 3.7)
surround the odontoid process of C2 vertebra in posterior, posterolateral, and lateral aspects (arrowheads). CT images showing a curvilinear
or arc-shaped periodontoid calcification in the region of transverse ligament (arrows).
DISCUSSION
CDS is an uncommon but important condition
encountered by many specialties. Approximately
320 cases were found in the English literature. It
remains a clinicoradiological entity but with a widening
spectrum, characterised by locoregional features (e.g.,
neck pain, neck rigidity, headache, shoulder pain),
inflammatory response (pyrexia, leucocytosis, elevated
erythrocyte sedimentation rate, C-reactive protein
level), and calcium crystal deposition at and around
the atlantoaxial articulations evident as periodontoid
calcifications on CT.[1] [2] [4] [5] [6] Nonetheless there is no
consensus on diagnostic or inclusion criteria, hence
a wide variation of reported presentations, e.g., local
symptom onset varies as acute, subacute, chronic,
periodic or uncertain; pyrexia is present or absent;
inflammatory markers are normal or elevated; and rare
features such as meningeal signs or cervical myelopathy.
FUO is also rarely reported, mimicked by cases of prolonged evolution with relapses.[2] [4] The two cases
we describe shared a common course: both were elderly
patients with a prolonged severe infective illness that
could cause flare-up of underlying CPPD disease with
CDS, and evolve into FUO without overt localising
sources other than generalised deconditioning. Such a
clinical scenario for CDS is underreported.
The radiological part of CDS, calcium crystal deposition
evident on CT, is the cornerstone and present in virtually
every reported case. It may have led to better recognition
of CDS, even when asymptomatic, in patients with
CPPD disease.[4] [6] Nonetheless such CT findings may also
be common in the elderly people with no CPPD disease
history. The prevalence of “incidental” periodontoid
calcifications on CT has been reported in the United
States to be 34% in those aged ≥60 years, and 49% for
those aged ≥80 years.[7] Corresponding prevalences of 15%
and 24% have been reported in Japanese patients.[8] The prevalence of “concomitant” periodontoid calcifications
with or without neck symptoms in CPPD patients has
been reported as 51% to 63%.[4] [6] [8] These prevalence data in
non-CPPD and CPPD patients highlight the importance
of other criteria such as pyrexia or inflammatory markers
in making a diagnosis of CDS, especially in the elderly
people.[7]
The use of FDG to image infection and inflammation
is widely accepted.[9] It can localise occult sources and
delineate extent and severity. FDG hyperactivity at
and around atlantoaxial articulations is strong evidence
of an active inflammatory process in vivo, constituting
the metabolic (inflammatory) form of structural
abnormalities on imaging. To the best of our knowledge,
only six cases of CDS with PET-CT have been recently
reported (five in English, one in Dutch).[10] [11] [12] We report
two additional cases, highlighting a characteristic
pattern of FDG hyperactivity crowning the dens, best
shown on axial or coronal planes. Hyperactivity was of
a mild-to-moderate degree. We propose the presence of
periodontoid FDG hyperactivity (higher than adjacent
background activity), conforming to the “FDG-crowned
dens” pattern, to be included as one of the criteria for
CDS, equivalent to periodontoid calcifications on CT.
This notion awaits further research or more reported
CDS with PET-CT features.
With reference to other radionuclide studies, four CDS
cases have been published with positive technetium-99m
diphosphonate bone scintigraphy. The mechanism of
diphosphonate uptake is chemisorption onto bone surface
and calcium crystals. This should aptly correspond to
periodontoid calcifications and any subchondral bony
changes secondary to degeneration or inflammation,
thus giving only little additional information to CT
alone. Bone scintigraphy is nonetheless helpful to
detect polyarthropathy or polyostotic disease. There
was no reporting of gallium-67 in the literature on CDS.
Gallium-67 scintigraphy was conventionally used to
investigate FUO.[13] Nonetheless its spatial resolution and
contrast sensitivity are poorer than that of PET-CT. This
may explain the negative gallium-67 scintigraphy in
the above cases despite a subsequent positive PET-CT,
particularly when the degree of inflammation during
asepsis was not as florid as that due to septic arthritis.
The aetiology of CDS is calcium pyrophosphate
(CPP) and/or basic calcium phosphate (BCP, mostly
hydroxyapatite) crystal deposition. CPP crystals
preferentially deposit in articulations (synovial fluid, hyaline cartilage, fibrocartilage, ligament, synovium,
capsule). BCP crystals are frequent in articular and
extra-articular tissue, especially tendons and soft tissue.
At the atlantoaxial region, CPP crystals deposit in
periodontoid structures, most frequently in transverse
ligament of the atlas posterior to dens of the axis,
whereas BCP crystals may also deposit in the longus
colli tendon anterior to dens. CDS is most frequently
due to CPPD disease that has a broad spectrum including
asymptomatic CPPD disease in the elderly people,
osteoarthritis with CPPD, acute CPP crystal arthritis
(formerly referred to as pseudogout), and chronic CPP
crystal inflammatory arthritis.[3] Nonetheless CDS can
also be related to hydroxyapatite deposition disease
that more often affects adult females with a favourable
outcome of calcium resorption,[14] or be reported in
rheumatoid arthritis, seronegative spondyloarthritis,
systemic sclerosis, and osteoarthritis.[15] Therefore, CDS
is not pathognomonic of CPPD disease and further
exploration of articular and peri-/extra-articular sites
is required to determine the underlying disease. FDG
PET-CT can evaluate inflammatory lesions in the body
and help direct further investigation at the most severe
and accessible sites and may differentiate CDS from
other conditions. Lastly, imaging methods per se do
not establish with absolute certainty the type of crystal
involved. A definitive diagnosis of CPPD disease is
based on the presence of CPP crystals in synovial fluid
or biopsied tissue.
Differential diagnoses for CDS encompass meningitis,
epidural abscess, cervical spondyloarthritis, polymyalgia
rheumatica, temporal arteritis, and neoplastic or
metastatic disease.[2] [4] Prior injury, surgery, and severe
intercurrent illness may cause underlying CPPD disease
to flare up.[3] The clinician should be constantly vigilant
for preceding or concomitant infection. In many reported
cases, a timely diagnosis of CDS can avoid invasive
investigations such as lumbar puncture, temporal artery
biopsy, or surgical exploration. CDS should typically
demonstrate dramatic improvement when treated with
non-steroidal anti-inflammatory drugs or colchicine and,
if clinically indicated, low-dose corticosteroids. An early
diagnosis of CDS is thus important to allow effective
treatment and avoid prolonged hospitalisation.
CONCLUSION
CDS remains an uncommon clinicoradiological entity
with a wide spectrum of presentations including FUO. CT
has been the cornerstone of diagnostics but periodontoid
calcifications per se may not always be associated with inflammation and may be prevalent in extreme elderly
people without disease. The characteristic metabolic
pattern of “FDG-crowned dens” is rarely recognised
but is strong evidence of active inflammation in vivo,
thereby helping to confirm CDS and leading to effective
treatment. We propose that this imaging pattern be
included as one of the criteria for CDS to heighten
physician awareness. With increasing use of PET-CT
for FUO, more cases may be detected, although whether
this will help refine diagnostic criteria or define subsets
awaits further research.
REFERENCES
1. Bouvet JP, le Parc JM, Michalski B, Benlahrache C, Auquier L.
Acute neck pain due to calcifications surrounding the odontoid
process: the crowned dens syndrome. Arthritis Rheum.
1985;28:1417-20. Crossref
2. Aouba A, Vuillemin-Bodaghi V, Mutschler C, De Bandt M.
Crowned dens syndrome misdiagnosed as polymyalgia rheumatica,
giant cell arteritis, meningitis or spondylitis: an analysis of eight
cases. Rheumatology (Oxford). 2004;43:1508-12. Crossref
3. Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition
disease. N Engl J Med. 2016;374:2575-84. Crossref
4. Salaffi F, Carotti M, Guglielmi G, Passarini G, Grassi W.
The crowned dens syndrome as a cause of neck pain: clinical
and computed tomography study in patients with calcium
pyrophosphate dihydrate deposition disease. Clin Exp Rheumatol.
2008;26:1040-6.
5. Godfrin-Valnet M, Godfrin G, Godard J, Prati C, Toussirot E,
Michel F, et al. Eighteen cases of crowned dens syndrome:
Presentation and diagnosis. Neurochirurgie. 2013;59:115-20. Crossref
6. Haikal A, Everist BM, Jetanalin P, Maz M. Cervical CT-dependent
diagnosis of crowned dens syndrome in calcium pyrophosphate
dihydrate crystal deposition disease. Am J Med. 2019;133:e32-7. Crossref
7. Chang EY, Lim WY, Wolfson T, Gamst AC, Chung CB, Bae WC,
et al. Frequency of atlantoaxial calcium pyrophosphate dihydrate
deposition at CT. Radiology. 2013;269:519-24. Crossref
8. Kobayashi T, Miyakoshi N, Konno N, Ishikawa Y, Noguchi H,
Shimada Y. Age-related prevalence of periodontoid calcification
and its associations with acute cervical pain. Asian Spine J.
2018;12:1117-22. Crossref
9. Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D,
Donohoe KJ, et al. EANM/SNMMI guideline for 18F-FDG use
in inflammation and infection. J Nucl Med. 2013;54:647-58. Crossref
10. Monet A, Massonnat R, Merino B, Riviere A, Richez C. Crowned
dens syndrome diagnosed on 18F-FDG PET/CT. Clin Nucl Med.
2014;39:1041-2. Crossref
11. Duizer ML, Hermsen R, Te Boekhorst T, Janssen S. Acute headache
and neck pain caused by crowned dens syndrome [in Dutch]. Ned
Tijdschr Geneeskd. 2018;162:D2699.
12. Ma J, Gonem R, Lever E, Stratton R, Singh A. SAT0436 Crowned-dens
syndrome: A recent case series in a single centre in The United
Kingdom. Ann Rheum Dis. 2019;78:1307-8. Crossref
13. Palestro CJ, Brown ML, Forstrom LA, Greenspan BS, McAfee JG,
Royal HD, et al. Society of nuclear medicine procedure guideline
for gallium scintigraphy in inflammation. Version 3.0, approved
June 2, 2004. Available from: https://s3.amazonaws.com/rdcms-snmmi/
files/production/public/docs/Gallium_Scintigraphy_in_Inflammation_v3.pdf. Accessed 1 Aug 2019.
14. Malca SA, Roche PH, Pellet W, Combalbert A. Crowned dens
syndrome: a manifestation of hydroxy-apatite rheumatism. Acta
Neurochir (Wien). 1995;135:126-30. Crossref
15. Scutellari PN, Galeotti R, Leprotti S, Ridolfi M, Franciosi R,
Antinolfi G. The crowned dens syndrome. Evaluation with CT
imaging [in Italian]. Radiol Med. 2007;112:195-207. Crossref