Prognostic Factors and Survival in Advanced Large Hepatocellular Carcinomas Treated with Combined Transarterial Chemoembolisation and Hypofractionated Image-guided Radiotherapy
ORIGINAL ARTICLE CME
Prognostic Factors and Survival in Advanced Large
Hepatocellular Carcinomas Treated with Combined Transarterial
Chemoembolisation and Hypofractionated Image-guided
Radiotherapy
NSM Wong1, CL Chiang1,2,3, CHM Ho1, WWL Yip1, CSY Yeung1, MKH Chan4, VWY Lee1, FAS Lee1, FCS Wong1
1 Department of Clinical Oncology, Tuen Mun Hospital, Tuen Mun, Hong Kong
2 Department of Clinical Oncology, The University of Hong Kong, Pokfulam, Hong Kong
3 Department of Clinical Oncology, The University of Hong Kong–Shenzhen Hospital, Shenzhen, China
4 Department of Radiation Physics, University Hospital Essen, Germany
Correspondence: Dr NSM Wong, Department of Clinical Oncology, Tuen Mun Hospital, Tuen Mun, Hong Kong. Email: wsm011@ha.org.hk
Submitted: 15 Sep 2019; Accepted: 17 Dec 2019.
Contributors: NSMW, CLC and FASL designed the study. NSMW, CLC, CHMH, WWLY, CSYY, MKHC, VWYL and FASL acquired the
data. NSMW and CLC analysed the data. NSMW and CLC drafted the manuscript. NSMW, FASL and FCSW critically revised the manuscript
for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was conducted in accordance with the Declaration of Helsinki, with approval from the New Territories West Cluster
Clinical & Research Ethics Committee (Ref NTWC/CREC/18064).
Abstract
Objectives
Large (≥10 cm) hepatocellular carcinomas (HCCs) carry a dismal prognosis and respond poorly to
transarterial chemoembolisation (TACE). Combined TACE and hypofractionated image-guided radiotherapy
(HIGRT) has emerged as a new treatment strategy. We evaluated its efficacy among these tumours and report the
predictors of overall survival (OS).
Methods
Data from 55 consecutive cases treated with preplanned combined TACE and HIGRT from 2007 to 2017
were evaluated from a prospectively collected database. Patients with advanced HCCs ≥10 cm, ineligible for curative
intervention and with Child-Pugh scores ≤B7, received one dose of preplanned TACE 4 weeks prior to HIGRT.
HIGRT doses were individualised according to the dose constraints of uninvolved liver and neighbouring organs
at risk. OS was the primary endpoint.
Results
In all, 55 patients with median tumour sizes of 15.3 cm were included. Tumour vascular thromboses and
extrahepatic diseases were present in 25.5% and 32.7%, respectively. The median total equivalent dose in 2 Gy/fr
(EQD2, α/β ratio = 10) was 32.7 Gy. The 2-year OS reached 24.9%. Clinical benefit rate was 83.6% with a 1-year
local control rate of 57.4%. Multivariate analyses revealed alpha-fetoprotein (AFP) level (hazard ratio = 2.2,
p = 0.025) and subsequent local treatment (hazard ratio = 0.2, p = 0.001) to be independent OS predictors. Responders
undergoing subsequent curative resection achieved significantly better median OS than those without.
Conclusion
Combined TACE and HIGRT achieved favourable survival outcomes among large HCCs. AFP level
and subsequent local surgery were independent negative and positive OS predictors, respectively. Future studies
are warranted.
Key Words: Carcinoma, hepatocellular; Chemoradiotherapy; Ethiodized oil; Prognostic factors
中文摘要
於晚期、大肝癌患者結合使用肝動脈化療栓塞法與影像導航之大分割
放射治療之預後因素與存活分析
黃善敏、蔣子樑、何凱文、葉穎鈴、楊善如、陳加慶、李蘊恩、李安誠、黃志成
目的
大型(不小於10公分)肝癌腫瘤預後尤為不良,對肝動脈化療栓塞法(TACE)的治療反應亦
更為遜色。TACE與影像導航大分割放射治療(HIGRT)的結合療法已漸獲初步認可。我們藉此評估
TACE及HIGRT於此類患者的結合運用之治療結果,並對總體存活期之預後因子作分析。
方法
本項研究以總體存活期為主要療效指標,於香港單一中心的前瞻性數據收集庫採納自2007至
2017年接受TACE與HIGRT結合治療的連續病例。當中包括合共55名晚期、大型(不小於10公分)、
不適合接受痊癒性手術切除、Child-Pugh不高於B7分級,以及於HIGRT的4週前曾接受一次性預先規
劃的TACE之肝癌患者。HIGRT劑量均按照未受累肝臟部分及鄰近危急器官之劑量限制而作個別調
整。
結果
共55名患者符合納入標準,其腫瘤大小中位數為15.3公分,而當中患有肝腫瘤血管栓塞和肝
外轉移則分別佔所有病例的25.5%及32.7%。以分次劑量(fr)2 Gy作計算,HIGRT的總處方劑量中位
數為32.7 Gy(EQD2,α/β ratio = 10)。治療患者的2年整體存活率達24.9%,而臨床獲益率及1年局部
控制率則分別達至83.6%和57.4%。多變項分析顯示高甲胎蛋白(AFP)水平(風險比值[HR] = 2.2,
p = 0.025)和療後的局部肝內治療(HR = 0.2,p = 0.001)分別屬於獨立的正面及負面總體存活期之
預後因素。其中療效理想因而成功接受根治性腫瘤切除之患者的總體存活期中位數更顯著超越未有
接受手術切除之患者。
結論
研究結果顯示TACE與HIGRT的結合治療於大肝癌腫瘤患者取得良好的存活成果。高AFP水平
及療後的局部肝內治療則分別為獨立的正面及負面總體存活期之預後因素。我們建議就此策略作進
一步研究。
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major global health
burden. According to the World Health Organization,
HCC ranked fifth in incidence and third in cancer
mortalities in 2018, in which incidence was highest
among East Asia.[1] During 2016, HCC was the fifth
commonest cancer and was the third commonest cause
of cancer-related deaths in Hong Kong.[2] The majority
of HCCs in Hong Kong are associated with hepatitis B
infection, whose carriers commonly present with sizable
tumours.[3] Large HCCs carry a dismal prognosis due to
their frequent associations with multiple satellite lesion
formation and vascular invasion.[4] [5]
Resection, radiofrequency ablation, and liver
transplantation provide the only chances of cure.
Unfortunately, only 30% of patients are candidates for
curative intervention at the time of presentation.[6] Among
unresectable tumours, transarterial chemoembolisation (TACE) is the most widely adopted locoregional
therapy.[7] [8] Randomised trials have demonstrated its
survival benefits over placebo.[9] [10] Efficacy is, however,
limited among those with large tumours or advanced
disease. Shim et al[11] reported the 2-year survival of HCC
patients receiving TACE was 42% versus 0% for tumour
sizes of 5 cm to 7 cm and ≥8 cm, respectively. The median
survival only reached 6 months among patients with
locally advanced disease treated with TACE according
to Yau et al.[12] This has illustrated the need for better
treatment strategies in patients with sizable tumours.
In light of technological advancements, stereotactic
body radiotherapy (SBRT) and/or hypofractionated
image-guided radiotherapy (HIGRT) have emerged
as promising local therapeutic options in patients
with localised HCCs. Several prospective series have
shown that SBRT was associated with encouraging
local control (LC) rates of 64% to 100% at 2 years, with limited toxicities.[13] [14] [15] [16] Growing evidence has also
demonstrated its effectiveness in patients with advanced
tumours.[17] Intriguingly, emerging data support the
potential synergistic effects of TACE and radiotherapy
(RT). Multiple reports have demonstrated combining
TACE and RT is associated with better outcome than
with single-modality treatment strategies.[18] [19] [20]
The aim of our study was to evaluate the efficacy and
safety of the combined TACE and HIGRT approach
among large unresectable HCCs ≥10 cm, as well as to
identify the predictive factors for overall survival (OS).
METHODS
Patients
This was a retrospective cohort of all patients treated
with combined TACE and HIGRT for unresectable
HCCs from 2007 to 2017 at Tuen Mun Hospital, Hong
Kong. Management strategies were determined by the
liver multidisciplinary team (MDT), in collaboration
with surgeons and radiologists. A radiological diagnosis
of HCC was made based on typical enhancement
patterns according to the dynamic imaging criteria of the
American Association for the Study of Liver Diseases.[8]
The criteria for treatment by combined TACE and HIGRT
were: (i) patients deemed unsuitable for resection, liver
transplantation, or local ablative therapies by the MDT;
(ii) tumour size ≥10 cm; (iii) a minimum of 700 mL of
uninvolved liver; (iv) an Eastern Cooperative Oncology
Group (ECOG) performance score ≤2; (v) a baseline
Child-Pugh (CP) liver score of A5 to B7; (vi) adequate
organ function, defined as absolute neutrophil count
≥1.5 × 109/L, creatinine ≥1.5 × upper limits of normal,
alanine aminotransferase or aspartate aminotransferase
<2.5 × upper limits of normal, and international
normalised ratio <1.7 without clinical evidence of ascites
or encephalopathy. Extrahepatic disease was allowed,
provided the greatest disease burden was intrahepatic.
Patients with portal vein thrombosis were also included.
Diffusely infiltrative HCCs were considered ineligible.
The Barcelona Clinic Liver Cancer (BCLC) stages
A to C were included according to its updated criteria
in which SBRT was also recommended as one of the
treatment options for stage A patients.[21]
Treatment
Transarterial Chemoembolisation
TACE was performed by superselective cannulation of the
artery supplying the tumour. The emulsion was prepared
by mixing ethiodised oil (lipiodol) with cisplatin in a 1:1 ratio by means of a pumping method.[22] The emulsion
was then injected slowly under fluoroscopic guidance
according to the size of the tumour and the arterial blood
flow.[23] One dose of TACE was administered 4 weeks
prior to HIGRT.
Hypofractionated Image-guided Radiotherapy
During the study period, various HIGRT techniques
were used. Patients were immobilised with a customised
device (Vac-Lok; MED-TEC, Orange City [IO], United
States). Computed tomography (CT) [Philips CT Big
Bore, Amsterdam, The Netherlands; 32 slices, helical
scan] with multiphasic intravenous contrast was used
to delineate the gross tumour volume (GTV). GTV was
contoured according to the areas containing lipiodol
and/or contrast enhancement as visualised on the planning
CT image. Breath-hold CT or 4-dimensional CT (average
phase or respiratory phase sorted) was used to determine
the internal target volume and/or planning target volume
(PTV). Motion management was done with maximum
intensity projection, gating, active breathing control, or
abdominal compression. RT was delivered by means of
dynamic conformal arc therapy (Varian Clinac 2100CD;
Varian Medical Systems, Palo Alto [CA], United States),
intensity-modulated RT, or volumetric modulated arc
therapy (Elekta beam modulator and Elekta Agility,
Stockholm, Sweden).
The total doses ranging from 4 Gy/fr to 6-10 frs, 5 fr/week, were individualised. The goal was to give the
highest possible dose with respect to normal tissue
constraints, in which the normal liver could receive
an equivalent dose in 2 Gy (EQD2, α/β ratio = 3) of
30 Gy <40% and mean dose <28 Gy. Dose constraints
to other organs at risk (OARs) included the small bowel,
stomach, large bowel, oesophagus, gallbladder, heart,
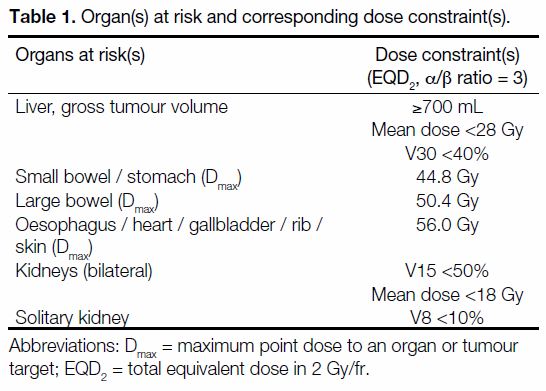
ribs, skin, and kidney(s) [Table 1].
Table 1. Organ(s) at risk and corresponding dose constraint(s).
Evaluation
Patients were assessed weekly during treatment, once
every 2 weeks for the first 2 months, then once for the
third month, followed by once every 3 months for the
first 2 years and every 4 months thereafter by the MDT.
Patients could also attend the oncology outpatient
clinic should they require further assistance. Physical
examination and liver function tests were performed on
every follow-up. A triphasic liver CT scan was obtained
at 3 months after HIGRT, every 3 months in the first year,
and every 6 months thereafter. The tumour response was
measured using Response Evaluation Criteria In Solid
Tumours (RECIST) criteria version 1.1.[24]
The primary endpoint of the study was OS. The secondary
endpoints were local in-field progression-free survival
(PFS), LC, response rate, toxicities, and prognostic
factors for OS. OS was calculated from the start of TACE
until the date of final follow-up or death. Local in-field
PFS was defined as the period from the date of starting
TACE to the time of local, in-field disease progression
or the time of patient death, whichever occurred first. LC
was defined as the absence of progressive disease within
the PTV. Patients with liver resection or transplantation
or radiofrequency ablation during follow-up were
censored for LC. A new lesion developing outside the
PTV was regarded as an intrahepatic out-of-field failure.
Toxicity was graded using the National Cancer Institute
Common Terminology Criteria for Adverse Events
version 5.0.[25] Acute adverse events (AEs) were defined
as AEs that occurred within 3 months after HIGRT. All
newly developed AEs or AEs that had progressed to
1 grade higher compared to baseline before treatment
were considered as AEs from HIGRT. Classic
radiation-induced liver disease (RILD) was defined as
an anicteric elevation in alkaline phosphatase of at least
twice the upper normal limit and non-malignant ascites
within 4 months after the completion of HIGRT.
Statistics
The LC, local in-field PFS, and OS results were evaluated
by means of Kaplan-Meier survival analysis. The log-rank
test was used to compare outcomes among survival
curves for identification of potential prognostic factors.
Any factors that were significant in univariate analyses
were subjected to multivariate analyses using the Cox
proportional hazards regression model. A p value <0.05
was considered statistically significant. SPSS (Windows
version 25.0; IBM Corp., Armonk [NY], United States)
was used for statistical analysis.
RESULTS
Patient and Treatment Characteristics
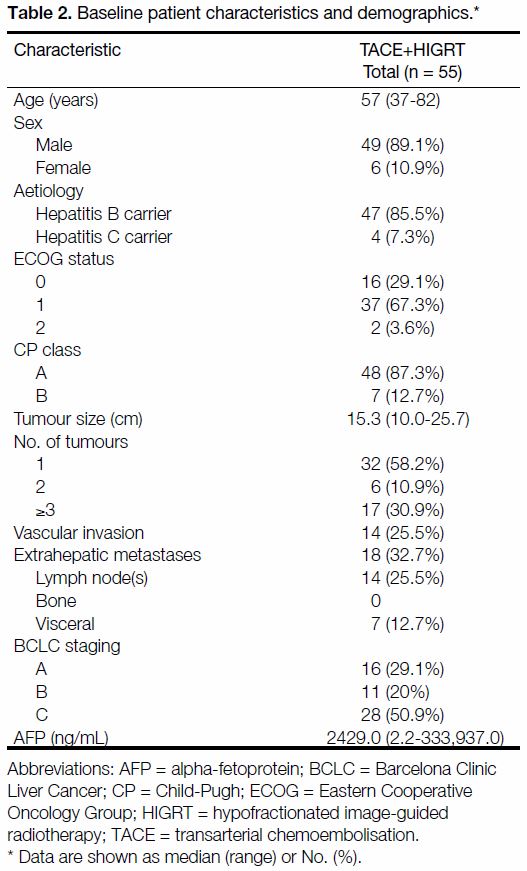
A total of 55 patients were included in the study. Patient
characteristics are listed in Table 2. The majority of
patients were male (89.1%), had ECOG status of 1
(67.3%), were hepatitis B carriers (85.5%), BCLC
stage C (50.9%), and with baseline median CP scores
of A5. One-third of the patients (32.7%) had baseline
extrahepatic metastases, 25.5% had vascular invasion and
41.8% had >1 baseline liver lesion. The median largest
tumour dimension was 15.3 cm (range, 10.0-25.7 cm) with
a median GTV of 1386.6 mL (range, 394.0-3990.7 mL).
Median serum alpha-fetoprotein (AFP) level was
2429 ng/mL (range, 2.2-333,937.0 ng/mL).
Table 2. Baseline patient characteristics and demographics.
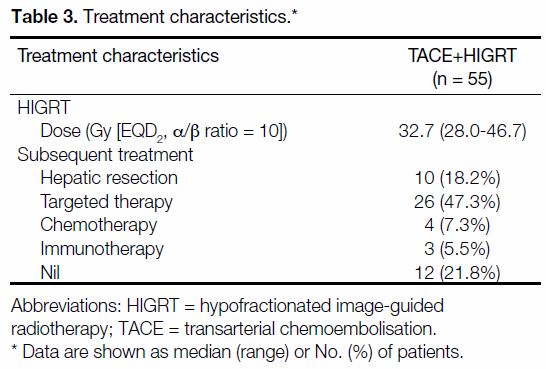
The median total equivalent dose (EQD2, α/β ratio = 10)
was 32.7 Gy (28 Gy in 7 fr). Subsequent local or systemic treatment was allowed, in which 18.2% (n = 10)
of patients underwent subsequent hepatic resection
with curative intent. A total of 47.3% (n = 26), 7.3%
(n = 4), and 5.5% (n = 3) subsequently received targeted
therapy, chemotherapy, or immunotherapy, respectively
(Table 3).
Table 3. Treatment characteristics.
Survival and Prognostic Factors
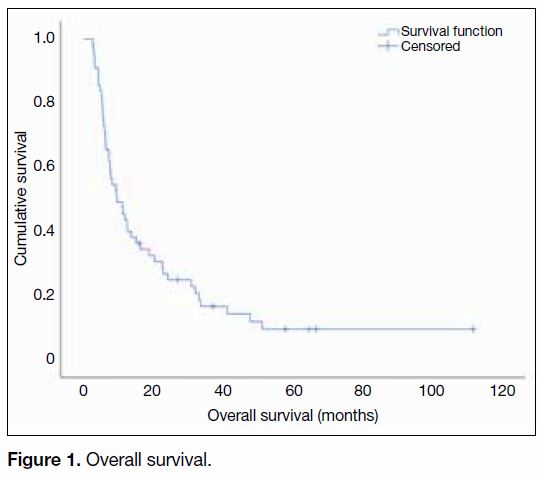
The median follow-up time for all patients was
8.5 months (range, 1.5-110.4 months). The median
OS was 9.5 months (95% confidence interval
[CI] = 5.2-13.7 months; range, 2.6-111.8 months) across
the studied population, with the 1-year and 2-year OS
reaching 43.6% (95% CI = 30.5%-56.7%) and 24.9%
(95% CI = 13.4%-36.4%), respectively (Figure 1).
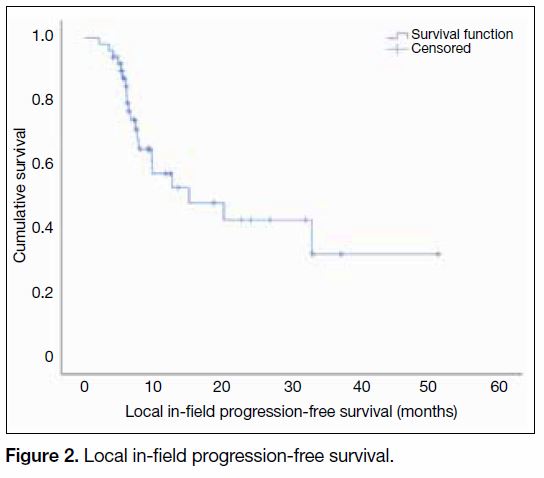
The median local in-field PFS reached 15.1 months
(95% CI = 2.1-28.1 months) [Figure 2].
Figure 1. Overall survival.
Figure 2. Local in-field progression-free survival.
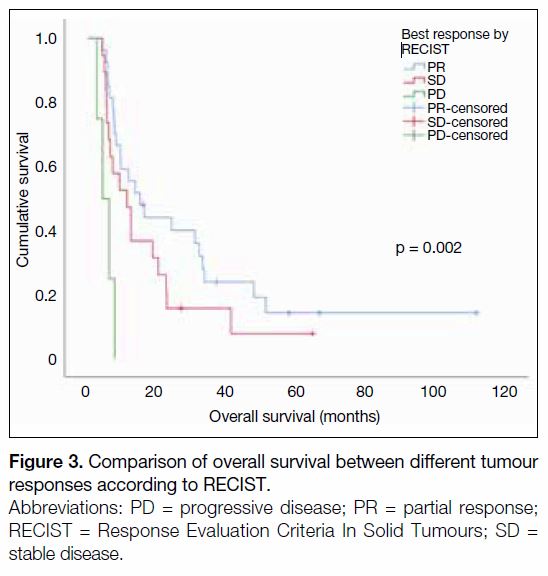
By means of Kaplan-Meier analysis, the best RECIST
treatment response was significantly associated with
OS (partial response [PR] vs. stable disease [SD] vs.
progressive disease, respectively: 15.0 vs. 11.2 vs.
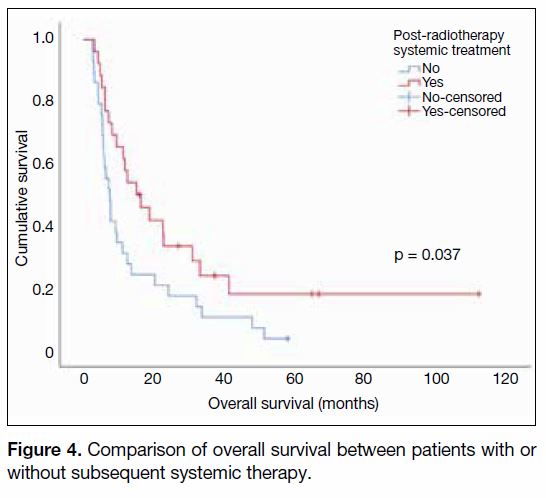
4.2 months, p = 0.002) [Figure 3]. It was also observed
that the presence of systemic treatment was associated
with better OS (15.0 vs. 7.5 months, p = 0.037)
[Figure 4].
Figure 3. Comparison of overall survival between different tumour
responses according to RECIST.
Figure 4. Comparison of overall survival between patients with or
without subsequent systemic therapy.
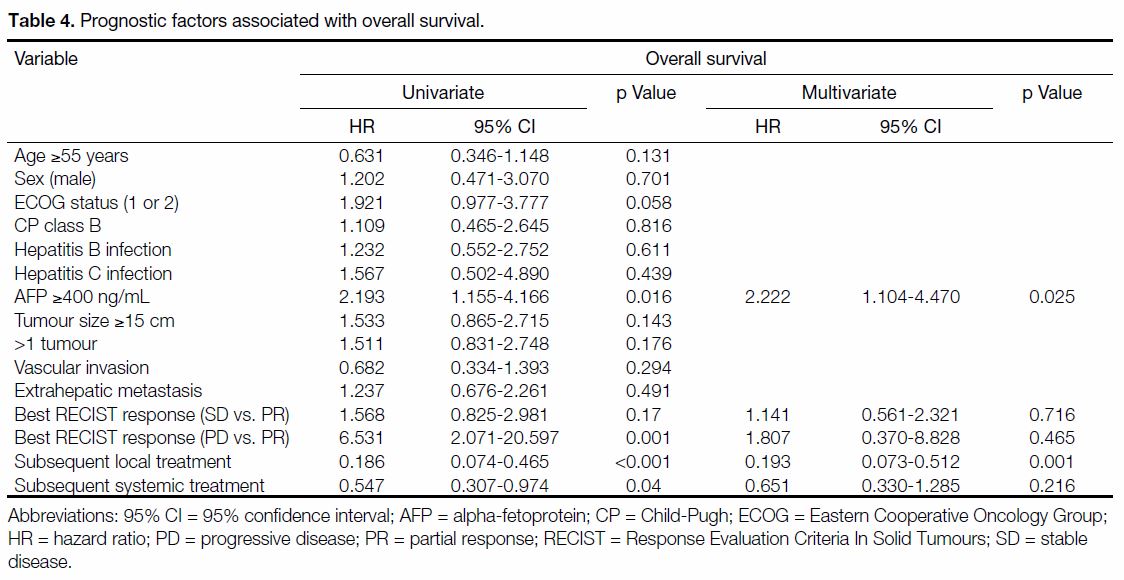
Univariate analysis revealed serum AFP (≥400 ng/mL),
treatment response according to RECIST criteria, and
subsequent local and systemic anti-cancer treatment
were significant prognostic factors for OS. On subsequent multivariate analysis, high AFP levels
(≥400 ng/mL) and the presence of subsequent local
anti-cancer treatment remained negative and positive
independent OS predictors, respectively (Table 4).
Table 4. Prognostic factors associated with overall survival.
Local Control and Pattern of Failure
The 1-year local, in-field control rate reached 57.4%
(95% CI = 40.8%-74.0%) and the 2-year LC rate
remained at 42.8% (95% CI = 23.9%-61.7%). The
clinical benefit rate (complete response [CR], PR,
and SD) according to RECIST 1.1 criteria was up to
83.6% (n = 46), of which 49% (n = 27) of the patients achieved PR and 34.5% (n = 19) attained SD. Of all
evaluable patients, 80% (n = 40, missing data = 5)
remained free from local progression at the time of
evaluation; 64% (n = 32, missing data = 5) developed
out-of-field intrahepatic progression; 2% (n = 1, missing
data = 4) developed vascular invasion, while 50% (n = 26,
missing data = 3) developed extrahepatic metastases.
Curative Treatment among Treatment
Responders
A total of 10 patients (18.2%) were able to undergo
subsequent curative resection following HIGRT. Of
those without baseline extrahepatic metastases, 27%
(10 out of 37) were successfully downstaged to undergo
curative resection. Eight (80%) had a baseline CP score
of A5 while two (20%) had a CP score B7, one (10%)
had vascular invasion and all were ECOG status 0-1 at
baseline. The EQD2, α/β ratio = 10 remained 32.7 Gy
(28 Gy in 7 frs) for the majority. Of the 10 final
pathological specimens, two (20%) had pathological
CR. Apart from one patient (10%) with an R1 resection,
all of the remaining (n = 9, 90%) achieved R0 resections.
Early postoperative mortality within 30 days was not
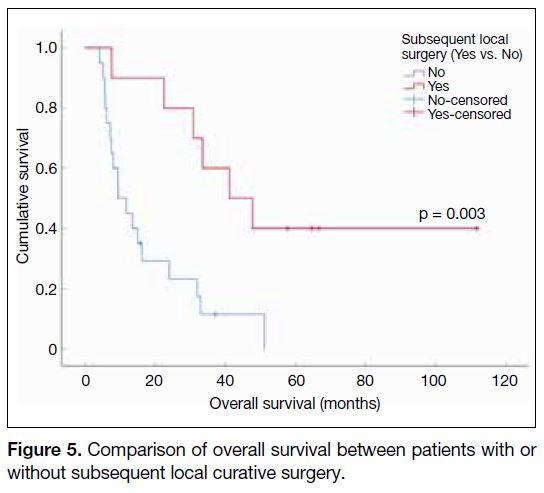
observed. The median OS among these patients reached
41.2 months (95% CI = 19.1-63.2 months), which was
significantly higher than those who did not undergo
surgery (9.5 months, 95% CI = 4.2-14.7 months, p = 0.003,
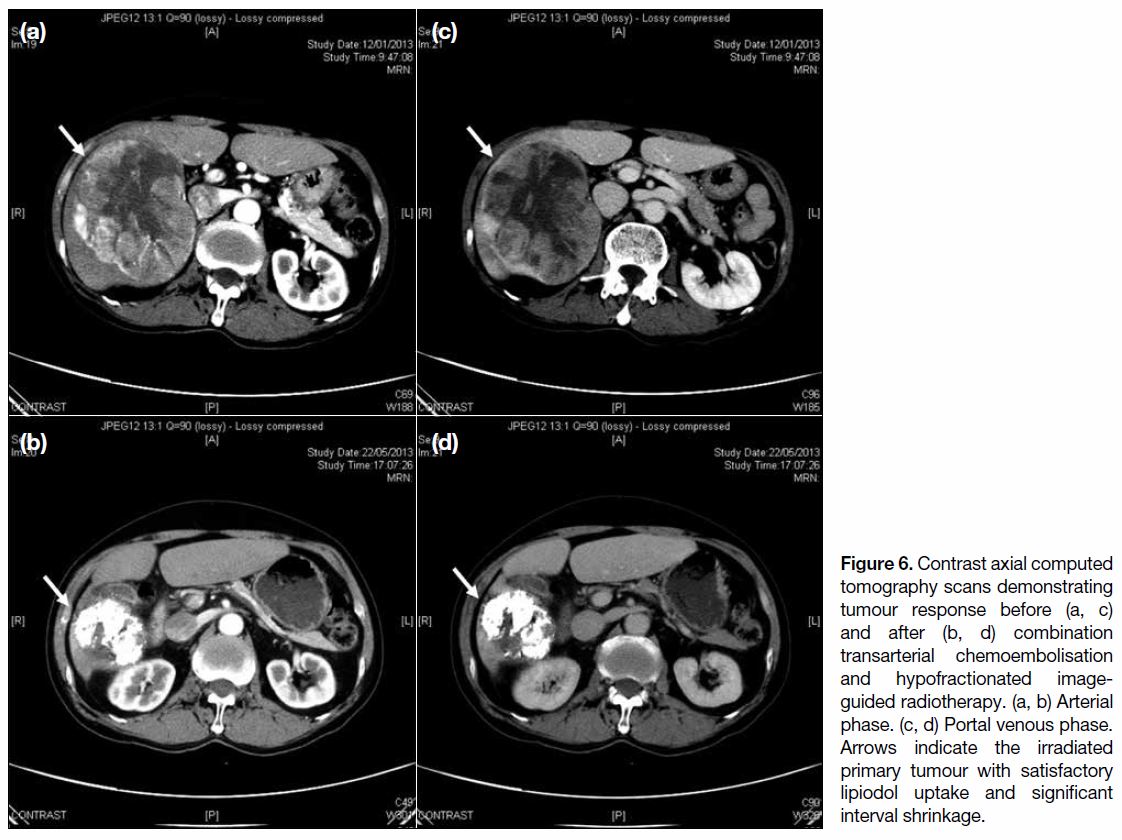
Figure 5). Figure 6 demonstrates tumour response to
combination TACE and HIGRT with marked interval
shrinkage.
Figure 5. Comparison of overall survival between patients with or
without subsequent local curative surgery.
Figure 6. Contrast axial computed
tomography scans demonstrating
tumour response before (a, c)
and after (b, d) combination
transarterial chemoembolisation
and hypofractionated image-guided
radiotherapy. (a, b) Arterial
phase. (c, d) Portal venous phase.
Arrows indicate the irradiated
primary tumour with satisfactory
lipiodol uptake and significant
interval shrinkage.
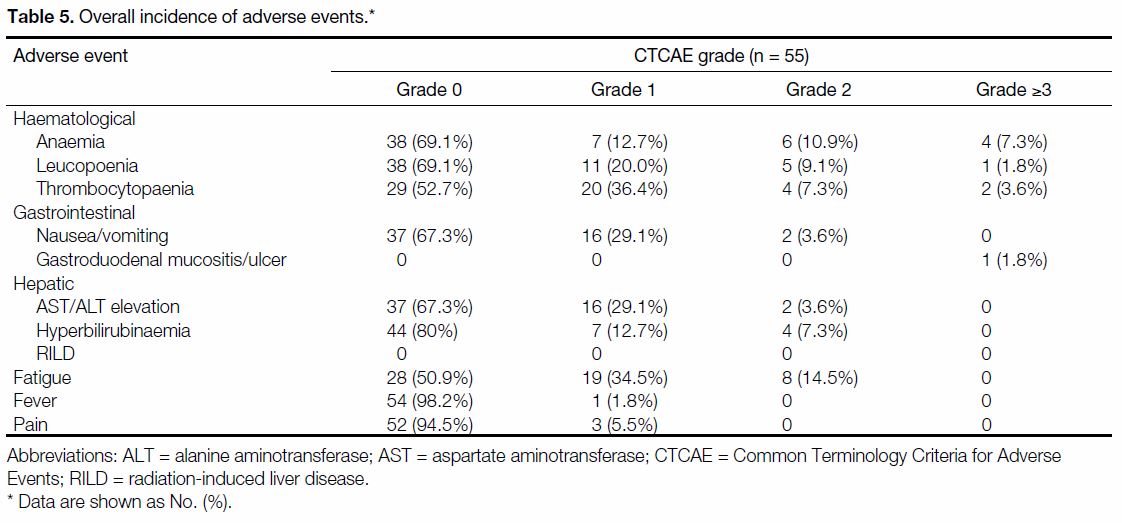
Adverse Events
Treatment completion rate was excellent at 94.5%, of
which the treatment delay/suspension rate was low at
12.7%. The rate of severe AEs, defined as Common
Terminology Criteria for Adverse Events grade ≥3, were
rare. One patient (1.8%) had grade 5 gastrointestinal
bleeding from the development of a gastric ulcer within
8 weeks post-RT. The remaining severe AEs were all
attributed to haematological toxicities (anaemia: n = 4,
7.3%; thrombocytopoenia: n = 2, 3.6% and neutropoenia:
n = 1, 1.8%). There were otherwise no other severe AEs
(Table 5).
Table 5. Overall incidence of adverse events.
DISCUSSION
This is the first study to report the role of combination
TACE and HIGRT among patients with HCCs ≥10 cm.
The baseline characteristics exhibited by our patients
reflected those with advanced disease status with a
substantial rate of high median baseline AFP exceeding
2000 ng/mL, vascular invasion (25.5%), extrahepatic
metastases (32.7%), and tumour multiplicity (41.8%),
which was consistent with reports describing the
aggressive disease nature, delayed presentation, and poor
prognoses of large tumours.[26] [27] Although BCLC class A
patients (according to the updated staging criteria)
were also included in the study, they were all deemed
unresectable by the MDT due to technical difficulties
such as unfavourable tumour position, inadequate liver
function, and medical risk.
There has always been an unmet need to improve the
poor survival outcomes of large or locally advanced
HCCs.[28] Our study population achieved a superior 2-year
OS of 24.9%, contrasting with Shim et al’s reporting of
a 0% 2-year OS for tumours ≥8 cm treated with TACE
alone,[11] suggesting a highly efficacious treatment
combination. It was also intriguing to observe patients
without baseline extrahepatic metastases, nearly 30% of
whom were able to undergo subsequent curative surgery
with a high R0 resection rate (two achieved pathological
CR) without early postoperative mortality, and in which
a translation to significant OS benefit was observed. A
high proportion (80%) of our patients remained free from
local progression at time of analysis further supports our hypothesis of an improved LC with such a combination
strategy.
An individualised dosage for HIGRT was pivotal. Mean
liver dose was reported to be independently associated
with CP score progression after RT, and that a mean
liver dose of >28 Gy EQD2, α/β ratio = 3 was associated
with a 5% risk of RILD.[29] [30] It has also been suggested
that the risk of liver function impairment following
TACE and RT was more common when V30 >40%
EQD2, α/β ratio = 3 among large HCCs.[31] In addition,
a study has suggested that fractionated RT could offer
dosimetric advantages over the 5-fr regimen among
large HCCs which were close to OARs.[32] It was therefore
justifiable that our institutional protocol adopted such
dose constraints and fractionated doses in tailoring RT
prescriptions. Large HCCs often limit dose escalation
due to their close proximities to surrounding OARs
with limited residual normal liver reserve. In our study,
treatment was well tolerated with a high completion rate approaching 95%. Severe (grade ≥3) AEs were rare, and
most were related to transient, reversible haematological
disturbances. Despite concerns for liver decompensation
following TACE and/or SBRT among HCC patients with
pre-existing compromised liver function, there were no
identifiable occurrences of classic RILD or of severe liver
function derangement. We have hereby demonstrated the
safety and feasibility of our RT regimen in combination
with pre-RT TACE for large HCCs.
Despite the modest prescribed RT doses (median
EQD2, α/β ratio = 10, 32.7 Gy, range, 28.0-46.7 Gy)
in our study, high 1-year LC and clinical benefit rates
were observed. Multiple studies have demonstrated
that combined TACE and RT are associated with better
outcome than either treatment alone.[19] [20] A previous
meta-analysis also demonstrated that a TACE-RT
interval <4 weeks was associated with better tumour
response compared to longer intervals.[18] We suggest that
prior chemoembolisation reduces viable tumour burden, thereby enhancing the therapeutic effect of a lower
RT dose for better LC. Future studies exploring the
underlying mechanisms of possible augmented effects of
TACE and HIGRT would yield invaluable information.
Multivariate analysis has identified baseline AFP
≥400 ng/mL and subsequent local treatment as
independent prognostic factors for OS. AFP has been
recognised as a poor prognostic factor for HCC, in which
a cut-off of 400 ng/mL has been included in the Cancer
of Liver Italian Program scoring system.[33] The system
was reported to be a good predictor of recurrence based
on a respective Chinese cohort of predominantly patients
with hepatitis B following curative surgery for HCC.[34]
In spite of achieving durable LC and high clinical benefit
rates among primary tumours treated with the combined
TACE and HIGRT approach, out-of-field intrahepatic
or extrahepatic progression remained mostly inevitable,
resulting in a precipitous OS decline beyond 2 years. Our
data presented significant OS benefit among patients who
underwent curative surgery after combined TACE and
HIGRT, and there was also a trend towards improved
OS among patients with radiological response post-TACE and HIGRT according to the RECIST scores.
Taken together, future studies investigating the role of
combined TACE and HIGRT as a neoadjuvant strategy
among selected patients for upfront unresectable HCCs
are much anticipated.[35] Furthermore, a statistically
significant, lengthened OS with addition of systemic
therapy following TACE and HIGRT by Kaplan-Meier
and univariate analysis was identified. This has shed light
on future trials investigating the optimal combination
strategies with merging systemic therapies that were not
available at the time of investigation.
This study carries several limitations. This was a single-centre
study in which potential bias might have been
introduced. Our study spanned across a long period
of 10 years with use of three different RT techniques,
which might have influenced the treatment outcome.
A substantial proportion of our patients underwent
subsequent treatment; the impact of initial TACE
and HIGRT treatment might have been diminished.
Our sample size was modest, in which a larger scale,
multi-centre, prospective, randomised trial would
have facilitated a more comprehensive analysis of the
prognostic factors for OS. However, our study has the
unique advantage of having long-term, experienced
and consistent partnerships with MDTs, treatment
personnel, RT planning, and treatment facilities. As a result, heterogeneity in patient selection and overall
management strategies were minimised throughout
the treatment period. Lastly, some might suggest that
radioembolisation with 90yttrium (90Y)-tagged glass
(TheraSphere; MDS Nordion, Ottawa, Canada) or resin
(SIR-Spheres; Sirtex Medical, Lane Cove, Australia) is a
viable alternative among the studied population.[36] [37] For
instance, Salem et al[35] reported comparable outcomes
to TACE in terms of response rate (42%) and time-toprogression
(7.9 months) for a sample of 291 patients
with median tumour sizes of 7 cm.[37] Although 90Y
radioembolisation in smaller tumours (range, 2.3-3.7 cm)
has been investigated in a more recent Phase II trial by
Salem et al,[38] suggesting a significantly better time to
progression with 90Y (>26 months vs. 6.8 months), it
remains unclear whether radioembolisation could result
in comparable outcomes in terms of survival and safety
profile as demonstrated in our study with TACE and
HIGRT.
CONCLUSION
Combined TACE and HIGRT achieved favourable
survival outcomes and good local tumour control with
low toxicities among large HCCs. High AFP levels and
subsequent local surgery were independent negative
and positive OS predictors, respectively, by means
of multivariate analysis. Future prospective trials are
warranted to determine its optimal integration into local
and systemic therapies to ultimately combat these large,
aggressive HCCs carrying a distinctly dismal prognosis.
REFERENCES
1. International Agency for Research on Cancer. Liver. World
Health Organization. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. 2018. Accessed 7 May
2020.
2. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR
Government. Hong Kong Cancer Registry 2016. Available from:
https://www3.ha.org.hk/cancereg/. Accessed 29 Aug 2019.
3. Ng J, Wu J. Hepatitis B- and hepatitis C-related hepatocellular
carcinomas in the united states: Similarities and differences. Hepat
Mon. 2012;12:e7635. Crossref
4. Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection
in patients with large hepatocellular carcinoma larger than 10 cm
in diameter. J Am Coll Surg. 2002;194:592-602. Crossref
5. Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, et al.
Outcome after curative resection for a huge (≥10 cm) hepatocellular
carcinoma and prognostic significance of gross tumor classification.
Am J Surg. 2009;198:693-701. Crossref
6. Bruix J, Sherman M, Practice Guidelines Committee, American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma. Hepatology. 2005;42:1208-36. Crossref
7. European Association for the Study of the Liver; European
Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular
carcinoma. J Hepatol. 2012;56:908-43. Crossref
8. Bruix J, Sherman M, American Association for the Study of Liver
Diseases. Management of hepatocellular carcinoma: An update.
Hepatology. 2011;53:1020-2. Crossref
9. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT,
et al. Randomized controlled trial of transarterial lipiodol
chemoembolization for unresectable hepatocellular carcinoma.
Hepatology. 2002;35:1164-71. Crossref
10. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al.
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma:
a randomised controlled trial. Lancet. 2002;359:1734-9. Crossref
11. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local
radiotherapy as a complement to incomplete transcatheter arterial
chemoembolization in locally advanced hepatocellular carcinoma.
Liver Int. 2005;25:1189-96. Crossref
12. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT.
Development of Hong Kong liver cancer staging system with
treatment stratification for patients with hepatocellular carcinoma.
Gastroenterology. 2014;146:1691-700.e3. Crossref
13. Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE,
et al. Phase I feasibility trial of stereotactic body radiation
therapy for primary hepatocellular carcinoma. Clin Transl Oncol.
2010;12:218-25. Crossref
14. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J,
et al. Stereotactic body radiotherapy for primary hepatocellular
carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447-53. Crossref
15. Price TR, Perkins SM, Sandrasegaran K, Henderson MA, Maluccio MA,
Zook JE, et al. Evaluation of response after stereotactic body
radiotherapy for hepatocellular carcinoma. Cancer. 2012;118:3191-
8. Crossref
16. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al.
Stereotactic body radiation therapy for inoperable hepatocellular
carcinoma as a local salvage treatment after incomplete transarterial
chemoembolization. Cancer. 2012;118:5424-31. Crossref
17. Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of
stereotactic body radiotherapy for hepatocellular carcinoma: A
systematic review and meta-analysis of observational studies.
Radiother Oncol. 2019;131:135-44. Crossref
18. Huo YR, Eslick GD. Transcatheter arterial chemoembolization
plus radiotherapy compared with chemoembolization alone for
hepatocellular carcinoma a systematic review and meta-analysis.
JAMA Oncol. 2015;1:756-65. Crossref
19. Jacob R, Turley F, Redden DT, Saddekni S, Aal AK, Keene K, et al.
Adjuvant stereotactic body radiotherapy following transarterial
chemoembolization in patients with non-resectable hepatocellular
carcinoma tumours of ≥3 cm. HPB (Oxford). 2015;17:140-9. Crossref
20. Su TS, Lu HZ, Cheng T, Zhou Y, Huang Y, Gao YC, et al. Longterm
survival analysis in combined transarterial embolization
and stereotactic body radiation therapy versus stereotactic body
radiation monotherapy for unresectable hepatocellular carcinoma
>5cm. BMC Cancer. 2016;16:834. Crossref
21. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM,
et al. Diagnosis, staging, and management of hepatocellular
carcinoma: 2018 Practice Guidance by the American Association
for the Study of Liver Diseases. Hepatology. 2018;68:723-50. Crossref
22. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily
chemoembolization of hepatocellular carcinoma. Radiology.
1989;170:783-6. Crossref
23. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular
carcinoma. Gastroenterology. 2004;127:S179-S188. Crossref
24. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S,
Mandrekar S, et al. RECIST 1.1 — Update and clarification: from
the RECIST committee. Eur J Cancer. 2016;62:132-7. Crossref
25. Cancer Therapy Evaluation Program, Division of Cancer Treatment
& Diagnosis, National Cancer Institute, US Department of Health
and Human Services. Common Terminology Criteria for Adverse
Events (CTCAE) v5.0. 2017. Available from: https://ctep.cancer.
gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5.0.xlsx. Accessed 29 Aug 2019.
26. Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO,
Ikai I, et al. Tumor size predicts vascular invasion and histologic
grade: Implications for selection of surgical treatment for
hepatocellular carcinoma. Liver Transplant. 2005;11:1086-92. Crossref
27. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M,
Mariani L, et al. Predicting survival after liver transplantation in
patients with hepatocellular carcinoma beyond the Milan criteria: a
retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. Crossref
28. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage
hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-35. Crossref
29. Velec M, Haddad CR, Craig T, Wang L, Lindsay P, Brierley J, et al.
Predictors of liver toxicity following stereotactic body radiation
therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol
Phys. 2017;97:939-46. Crossref
30. Dawson LA, Ten Haken RK. Partial volume tolerance of the liver
to radiation. Semin Radiat Oncol. 2005;15:279-83. Crossref
31. Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y,
Yoden E, et al. Prospective trial of combined transcatheter
arterial chemoembolization and three-dimensional conformal
radiotherapy for portal vein tumor thrombus in patients with
unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol
Phys. 2003;57:113-9. Crossref
32. Dow J, Matuszak MM, Brock KK, Ten Haken RK, Balter J,
Lawrence TS, et al. Potential benefits of fractionation over
SBRT for large liver tumors. Int J Radiat Oncol Biol Phys.
2015;93(Suppl):E169-70. Crossref
33. A new prognostic system for hepatocellular carcinoma: a
retrospective study of 435 patients: the Cancer of the Liver Italian
Program (CLIP) investigators [editorial]. Hepatology. 1998;28:751-5. Crossref
34. Zhao WH, Ma ZM, Zhou XR, Feng YZ, Fang BS. Prediction of
recurrence and prognosis in patients with hepatocellular carcinoma
after resection by use of CLIP score. World J Gastroenterol.
2002;8:237-42. Crossref
35. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant
three-dimensional conformal radiotherapy for resectable
hepatocellular carcinoma with portal vein tumor thrombus: a
randomized, open-label, multicenter controlled study. J Clin Oncol.
2019;37:2141-51. Crossref
36. Chow PK, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al.
SIRveNIB: Selective internal radiation therapy versus sorafenib in
Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol.
2018;36:1913-21. Crossref
37. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK,
Ibrahim S, et al. Radioembolization for hepatocellular carcinoma
using yttrium-90 microspheres: a comprehensive report of long-term
outcomes. Gastroenterology. 2010;138:52-64. Crossref
38. Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al.
Y90 radioembolization significantly prolongs time to progression
compared with chemoembolization in patients with hepatocellular
carcinoma. Gastroenterology. 2016;151:1155-63.e2. Crossref