Local and Regional Staging of Nasopharyngeal Carcinoma Using Magnetic Resonance Imaging
PICTORIAL ESSAY
Local and Regional Staging of Nasopharyngeal Carcinoma Using Magnetic Resonance Imaging
KY Chan, JCW Siu
Department of Radiology, Tuen Mun Hospital, Tuen Mun, Hong Kong
Correspondence: Dr KY Chan, Department of Radiology, Tuen Mun Hospital, Tuen Mun, Hong Kong. Email: andrew_yuk@msn.com
Submitted: 15 Jan 2019; Accepted: 21 Mar 2019.
Contributors: KYC designed the study, acquired the data, and drafted the manuscript. All authors analysed and interpreted the data and critically
revised the manuscript for important intellectual content. All authors had full access to the data, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This pictorial essay received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the New Territories West Cluster Research Ethics Committee (Ref NTWC/REC/19113).
BACKGROUND
Nasopharyngeal carcinoma is the most common cancer
originating from the nasopharynx. The tumour accounts
for up to 70% of all primary malignant lesions arising
from the nasopharynx.[1] [2] Most cases are seen in adults
with the disease rare in the paediatric population. The
peak incidence occurs between age 40 and 60 years. The
disease is prevalent in southern China, with most cases
located in Guangdong, Guangxi, and Fujian provinces.[3]
The disease is relatively rare in Western populations.
The disease is more prevalent among men than women,
with the incidence in men up to 3 times that of women,[4]
regardless of geographic location.
Multiple aetiologies have been postulated in the
development of nasopharyngeal carcinoma. The first is
viral: the Epstein-Barr virus shows a strong association
in some cases of nasopharyngeal carcinoma.[5] A positive
correlation for level of antibodies to Epstein-Barr virus
(EBV) with size of tumour has been demonstrated, with
the former used to monitor disease status and response
to therapy. Recently, human papilloma virus has been
shown to have an association with non-endemic EBV
negative cases of nasopharyngeal carcinoma. These
cases demonstrate a worse outcome than EBV positive
cases.[6] A second aetiology is related to environmental factors. Chinese-style salted fish and other preserved
foods contain high levels of volatile nitrosamine, a
carcinogen that contributes to the development of many
cases of nasopharyngeal carcinoma. Other environmental
factors include smoking and cooking. Genetics also play
a role in the development of nasopharyngeal carcinoma:
multiple human leukocyte antigens have been shown to
have a causative effect.[7]
Similar to many other squamous cell carcinomas in the
head and neck region, the TNM staging system is applied
in nasopharyngeal carcinoma.[8] [9] This pictorial essay
focuses on different stages of nasopharyngeal carcinoma
according to the latest guideline from the American Joint
Committee on Cancer (AJCC) 8th edition, that has been
recommended and implemented since 1 January 2018.[8] [9]
Comment is made about revisions made since the 7th
edition. A series of nasopharyngeal carcinoma cases of
various stages was retrieved from the reporting system
of the Department of Radiology at Tuen Mun Hospital.
IMAGING FINDINGS
The typical appearance of nasopharyngeal carcinoma
is that of a T1 isointense and T2 isointense/mildly
hyperintense lesion with reference to the adjacent
muscles. It usually shows heterogeneous contrast enhancement following injection of gadolinium contrast,
particularly useful when evaluating perineural spread of
the disease. For both T1 post-contrast and T2-weighted
sequences, a fat saturation technique is usually employed
to improve visualisation of the tumour.[10]
T STAGE
Detailed descriptions of the local staging of tumours can
be found in the AJCC Cancer Staging Manual.[9]
TX or T0
TX indicates that the primary tumour is too small or
inconspicuous to be visualised on imaging. T0 is a
new addition to the AJCC 8th edition, and describes no
conspicuous primary tumour detected despite positive
EBV involvement of cervical lymph nodes.[11] It is
relatively uncommon among different T stages owing to
the high sensitivity of magnetic resonance imaging.[12]
T1 Disease
T1 disease indicates that the tumour is confined to the
nasopharynx, or has extended to the oropharynx and/or
nasal cavity without parapharyngeal involvement.[9] The
nasopharynx is defined as the most superior part of the
pharynx. Inferiorly, it continues as the oropharynx and
anteriorly as the nasal cavity through the choana. On
its superior aspect, it is bound by the basisphenoid and
basiocciput that form the roof of the nasopharynx. On the
bilateral lateral aspects of the nasopharynx, there is focal
insinuation of mucosa to form an indentation called the
fossa of Rosenmüller, a.k.a. lateral recess. It is the most
common site of origin for nasopharyngeal carcinoma.[13]
The fossa of Rosenmüller lies posterior to the opening of the eustachian tube, and torus tubarius, an elevation
at the base of the cartilaginous portion of the eustachian
tube. There is no involvement of adjacent prevertebral
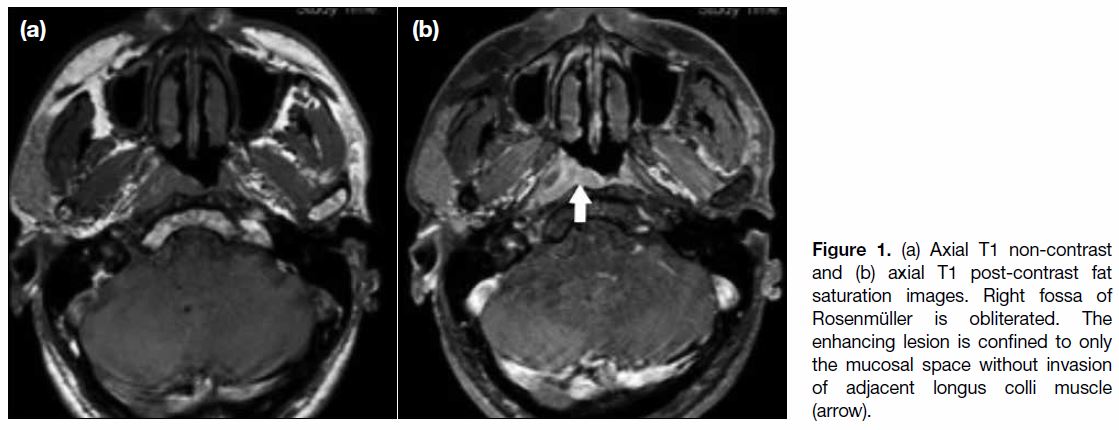
muscle or parapharyngeal space in T1 disease (Figure 1).
Figure 1. (a) Axial T1 non-contrast and (b) axial T1 post-contrast fat saturation images. Right fossa of Rosenmüller is obliterated. The enhancing lesion is confined to only the mucosal space without invasion of adjacent longus colli muscle (arrow).
Nasopharyngeal carcinoma tends to spread upwards
rather than inferiorly to the oropharynx,[12] [14] although
involvement of the oropharynx is still denoted as T1
disease (Figure 2). The boundary of the nasopharynx and oropharynx is defined according to the level of the
soft palate. Solitary involvement of the oropharynx is
uncommon as the tumour tends to spread upwards and
laterally to the parapharyngeal space.[12] [14] Nasopharyngeal
tumour extending to the nasal cavity or oropharynx but
not the parapharyngeal space has no significantly worse
outcome than tumours confined to the nasopharynx. As
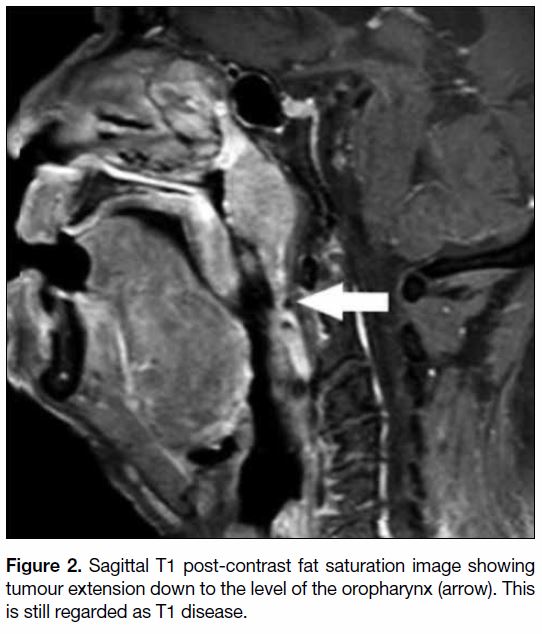
such they are both categorised as T1 disease.
Figure 2. Sagittal T1 post-contrast fat saturation image showing tumour extension down to the level of the oropharynx (arrow). This
is still regarded as T1 disease.
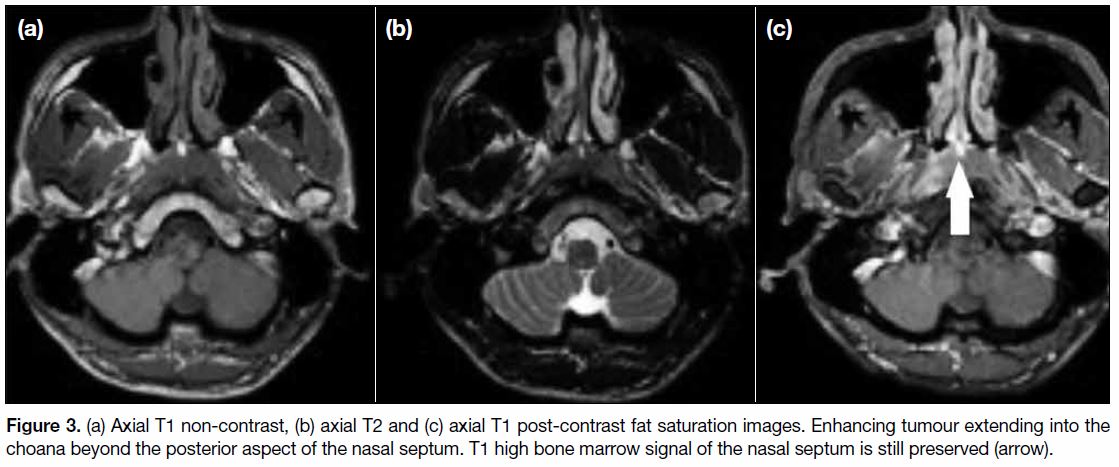
If the tumour extends beyond the choana, it enters the
nasal cavity. Usually there is only minimal extension
through the choana (Figure 3) and bulky tumour growth
within the nasal cavity is relatively uncommon.[12]
Expansion of the nasal cavity may be noted without definite invasion of underlying osseous structure as
evidenced by the preserved T1 fatty signal.[10]
Figure 3. (a) Axial T1 non-contrast, (b) axial T2 and (c) axial T1 post-contrast fat saturation images. Enhancing tumour extending into the
choana beyond the posterior aspect of the nasal septum. T1 high bone marrow signal of the nasal septum is still preserved (arrow).
The criteria for T1 disease in the AJCC 8th edition are
unchanged from the 7th edition.
T2 Disease
T2 disease is defined as tumour with extension into
the parapharyngeal space, and/or adjacent soft tissue
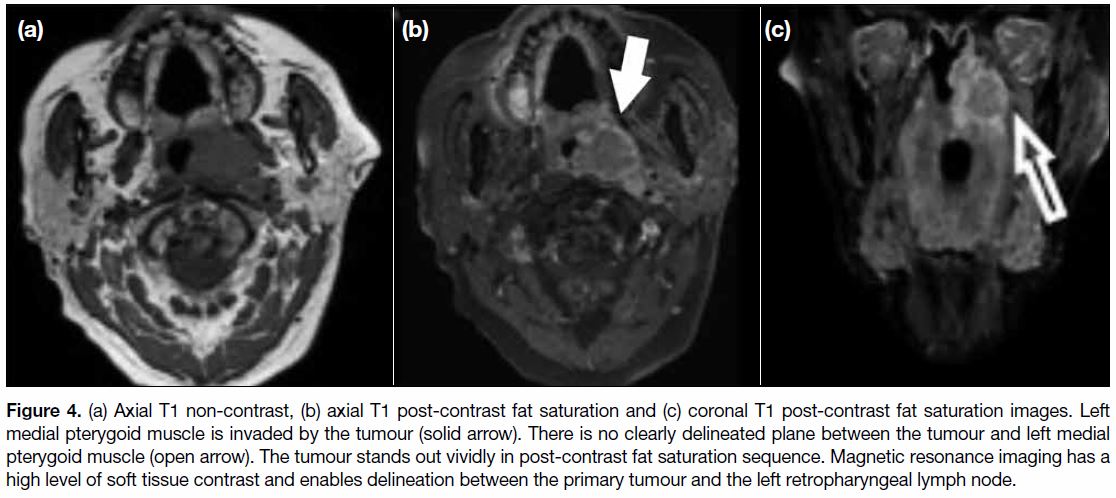
involvement including medial pterygoid (Figure 4),
lateral pterygoid and prevertebral muscle (Figure 5).[9]
In the AJCC 7th edition, involvement of the pterygoid
muscles was denoted as T4 disease.[11] The medial aspect
of the parapharyngeal space is bound by the levator veli palatini muscle that is an elevator muscle of the soft
palate. When the tumour invades through this muscle
laterally and the pharyngobasilar fascia (the submucosal
layer between mucosa and muscular layer) into the fatty
parapharyngeal space, it is categorised as T2 disease
(Figure 6). When the tumour invades the parapharyngeal
space, structures such as the ascending pharyngeal
artery, lymph nodes, part of pterygoid venous plexus and
branches of the trigeminal nerve may be affected.[15]
Figure 4. (a) Axial T1 non-contrast, (b) axial T1 post-contrast fat saturation and (c) coronal T1 post-contrast fat saturation images. Left
medial pterygoid muscle is invaded by the tumour (solid arrow). There is no clearly delineated plane between the tumour and left medial
pterygoid muscle (open arrow). The tumour stands out vividly in post-contrast fat saturation sequence. Magnetic resonance imaging has a
high level of soft tissue contrast and enables delineation between the primary tumour and the left retropharyngeal lymph node.
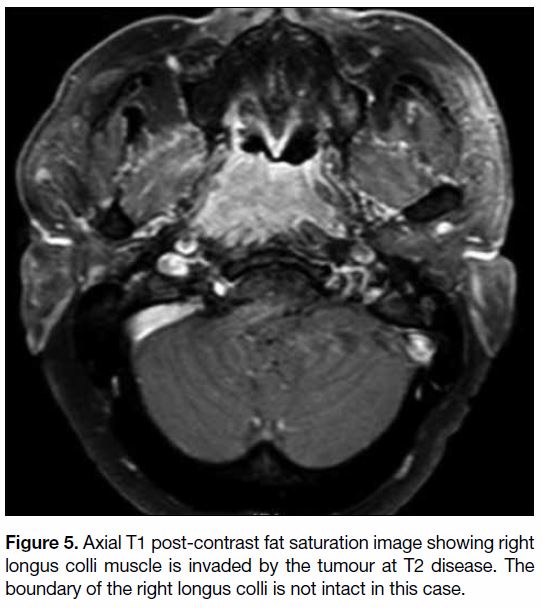
Figure 5. Axial T1 post-contrast fat saturation image showing right
longus colli muscle is invaded by the tumour at T2 disease. The
boundary of the right longus colli is not intact in this case.
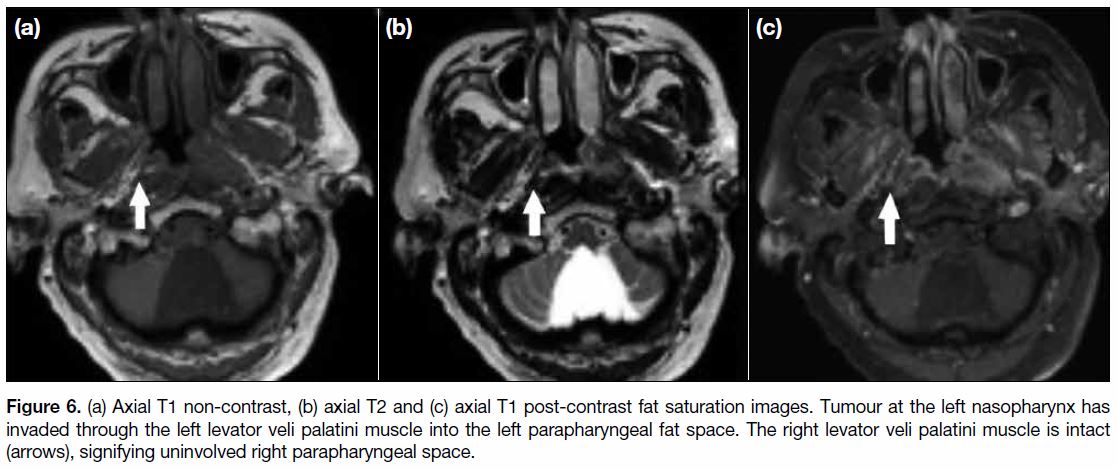
Figure 6. (a) Axial T1 non-contrast, (b) axial T2 and (c) axial T1 post-contrast fat saturation images. Tumour at the left nasopharynx has
invaded through the left levator veli palatini muscle into the left parapharyngeal fat space. The right levator veli palatini muscle is intact
(arrows), signifying uninvolved right parapharyngeal space.
With T2 disease, the tumour is usually quite sizable and often compresses the Eustachian tube with consequent
complications such as mastoid effusion (Figure 7).
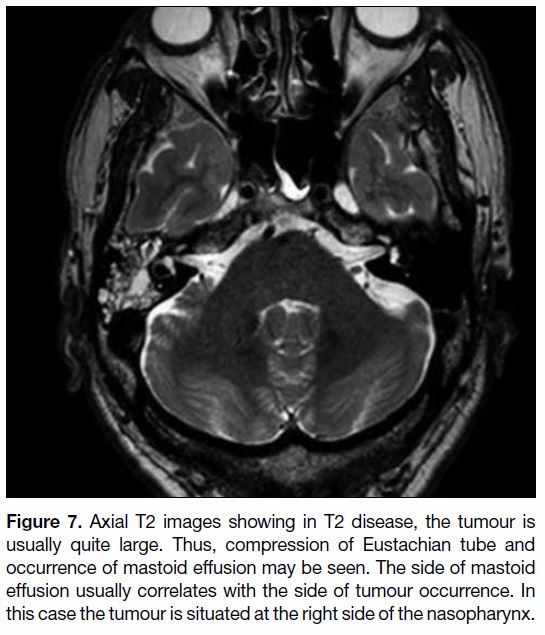
Figure 7. Axial T2 images showing in T2 disease, the tumour is
usually quite large. Thus, compression of Eustachian tube and
occurrence of mastoid effusion may be seen. The side of mastoid
effusion usually correlates with the side of tumour occurrence. In
this case the tumour is situated at the right side of the nasopharynx.
Adjacent soft tissue involvement including the medial
pterygoid, lateral pterygoid or prevertebral muscles
have been added to T2 classification in addition to
parapharyngeal involvement in the AJCC 8th edition.
T3 Disease
T3 disease is defined as tumour with infiltration of bony
structures at the skull base, cervical vertebra, pterygoid
structures, and/or paranasal sinuses.[9] The most common
bony structures involved include the clivus (Figure 8), the pterygoid bones, petrous apices and body of
sphenoid. Bony invasion can be readily confirmed
through evaluation of any loss of T1 fatty marrow signal
and comparison of bilateral bony structures.[9]
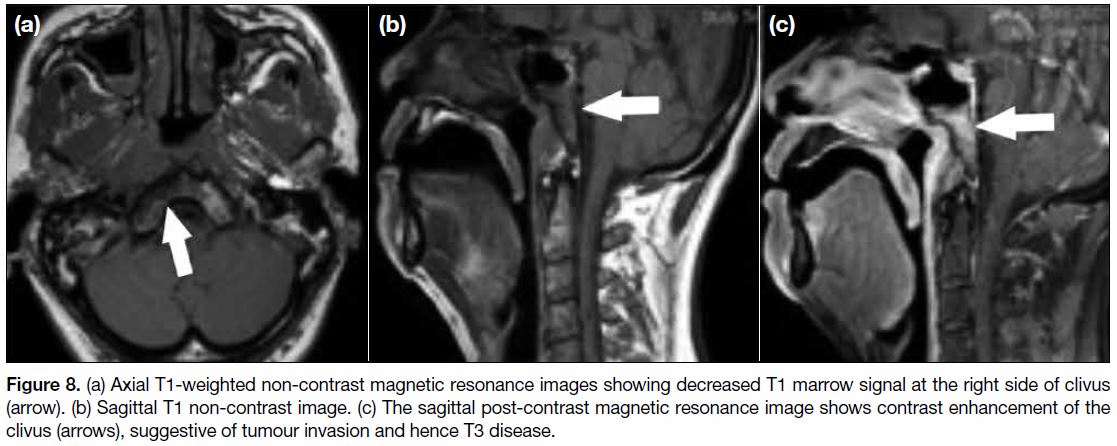
Figure 8. (a) Axial T1-weighted non-contrast magnetic resonance images showing decreased T1 marrow signal at the right side of clivus
(arrow). (b) Sagittal T1 non-contrast image. (c) The sagittal post-contrast magnetic resonance image shows contrast enhancement of the
clivus (arrows), suggestive of tumour invasion and hence T3 disease.
A couple of skull base foramina and canal are also
evaluated, including the foramen lacerum, foramen ovale,
foramen spinosum, foramen rotundum, carotid canal,
jugular foramen, and hypoglossal canal. As most of these
skull base foramina enable passage of neurovascular
structures craniocaudally, optimal evaluation uses the
coronal plane that has the added benefit of enabling
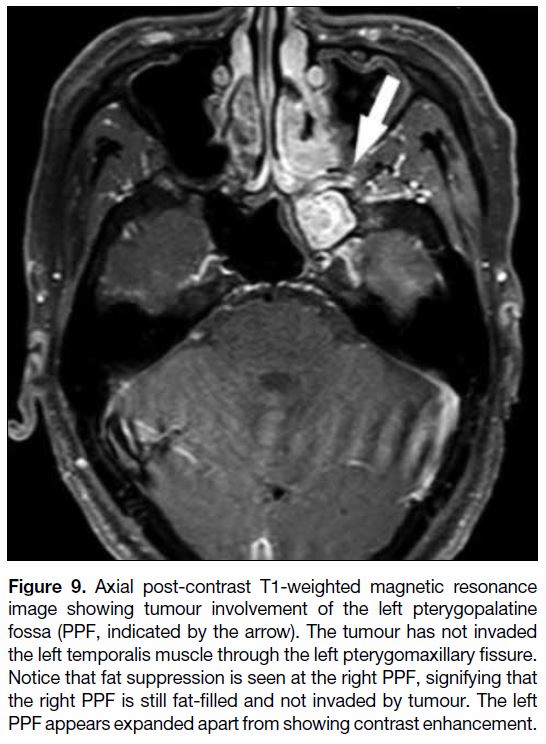
bilateral structural comparison. Involvement of the
pterygopalatine fossa is commonly denoted as T3 and
evaluation of this structure is of utmost importance in
staging nasopharyngeal carcinoma as this structure
is cross-roads in head and neck imaging (Figure 9).
Medially it communicates with the nasal cavity via the
sphenopalatine foramen. Laterally it communicates with
the masticator space via the pterygomaxillary fissure.
Figure 9. Axial post-contrast T1-weighted magnetic resonance
image showing tumour involvement of the left pterygopalatine
fossa (PPF, indicated by the arrow). The tumour has not invaded
the left temporalis muscle through the left pterygomaxillary fissure.
Notice that fat suppression is seen at the right PPF, signifying that
the right PPF is still fat-filled and not invaded by tumour. The left
PPF appears expanded apart from showing contrast enhancement.
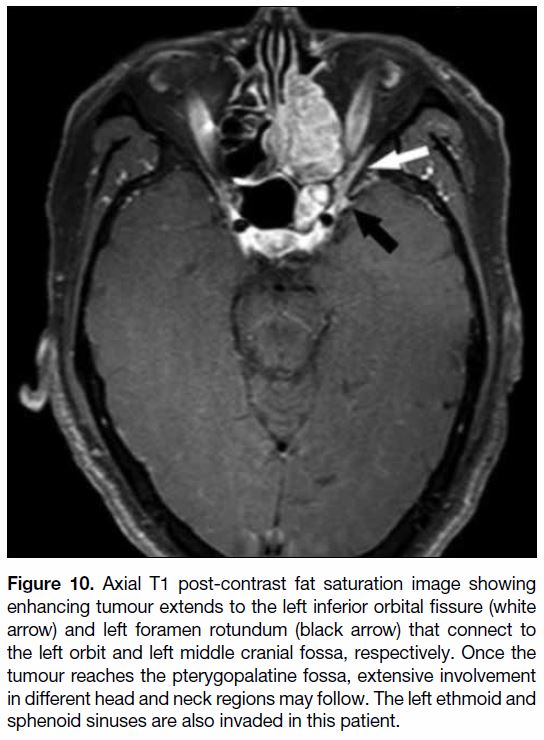
Anteriorly it continues into the orbit through the inferior
orbital fissure (Figure 10). Posteriorly and superiorly, it
communicates with the cavernous sinuses of the middle
cranial fossa through the foramen rotundum (Figure 10).
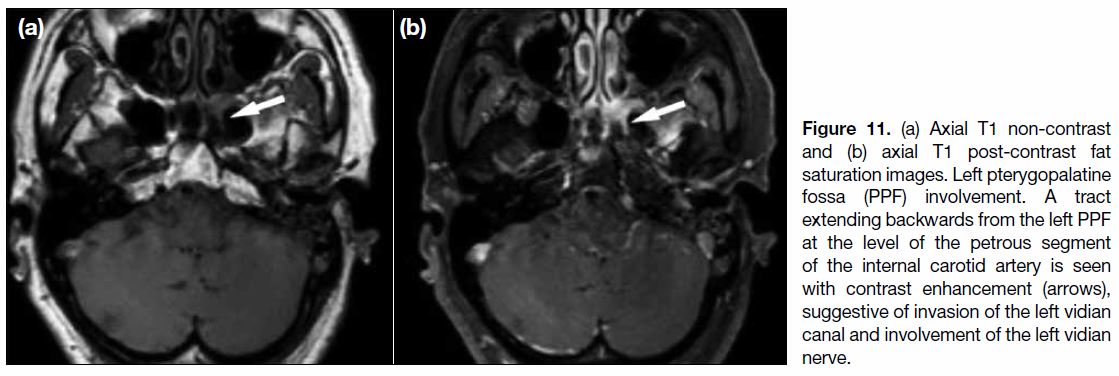
Posteriorly and inferiorly, another passage into the middle
cranial fossa is through the vidian canal (Figure 11).
Posteriorly and medially, it communicates with the
nasal cavity via the palatovaginal canal. Inferiorly,
it continues into the palate via the greater and lesser
palatine canals.
Figure 10. Axial T1 post-contrast fat saturation image showing
enhancing tumour extends to the left inferior orbital fissure (white
arrow) and left foramen rotundum (black arrow) that connect to
the left orbit and left middle cranial fossa, respectively. Once the
tumour reaches the pterygopalatine fossa, extensive involvement
in different head and neck regions may follow. The left ethmoid and
sphenoid sinuses are also invaded in this patient.
Figure 11. (a) Axial T1 non-contrast and (b) axial T1 post-contrast fat saturation images. Left pterygopalatine fossa (PPF) involvement. A tract extending backwards from the left PPF at the level of the petrous segment of the internal carotid artery is seen with contrast enhancement (arrows), suggestive of invasion of the left vidian canal and involvement of the left vidian nerve.
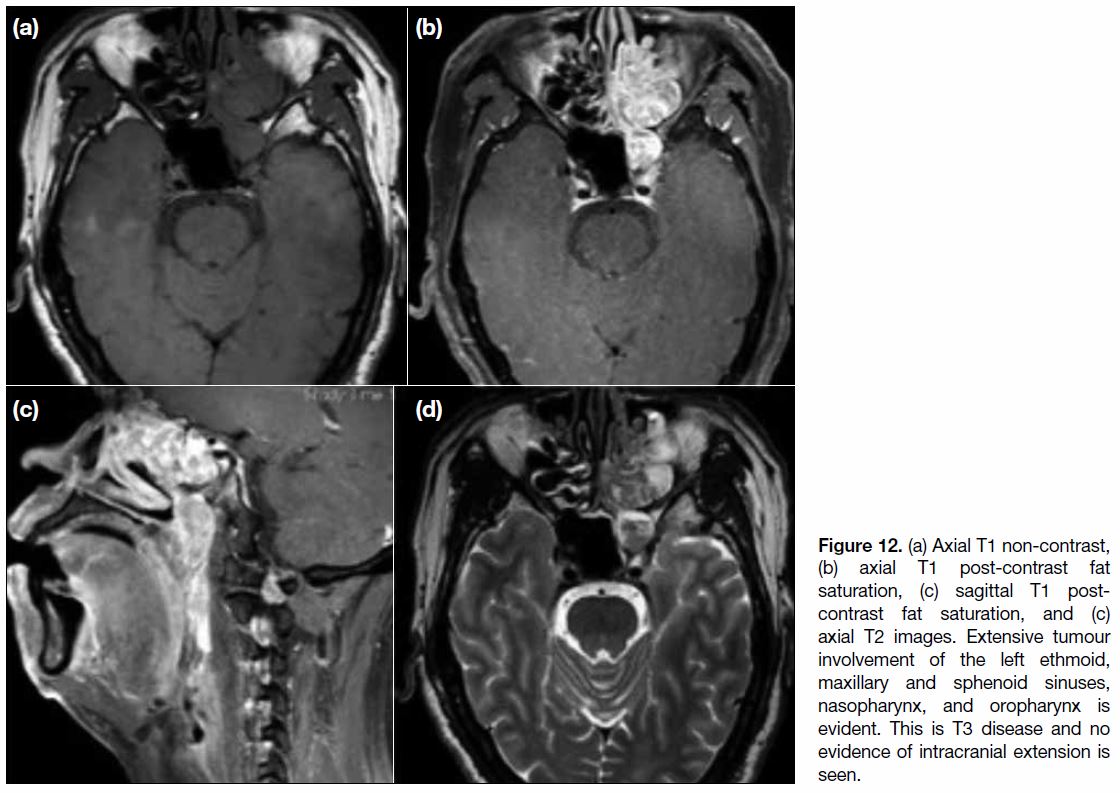
When the tumour invades the paranasal sinuses, the
disease is quite advanced (Figure 12). Continuity
between a mass at the nasopharynx and paranasal sinus
disease involvement is often observed. Absence of
metastasis to the paranasal sinuses is rare.[9] [12] When a
solitary paranasal sinus tumour is seen, another primary
with differential diagnoses including sinonasal squamous
cell carcinoma or sinonasal undifferentiated carcinoma
should be considered.[16]
Figure 12. (a) Axial T1 non-contrast, (b) axial T1 post-contrast fat saturation, (c) sagittal T1 post-contrast fat saturation, and (c) axial T2 images. Extensive tumour involvement of the left ethmoid, maxillary and sphenoid sinuses, nasopharynx, and oropharynx is evident. This is T3 disease and no evidence of intracranial extension is seen.
In T3 disease, further specification of bony structure
involvement including the skull base, cervical vertebra
and pterygoid structures has been added to the AJCC 8th
edition.
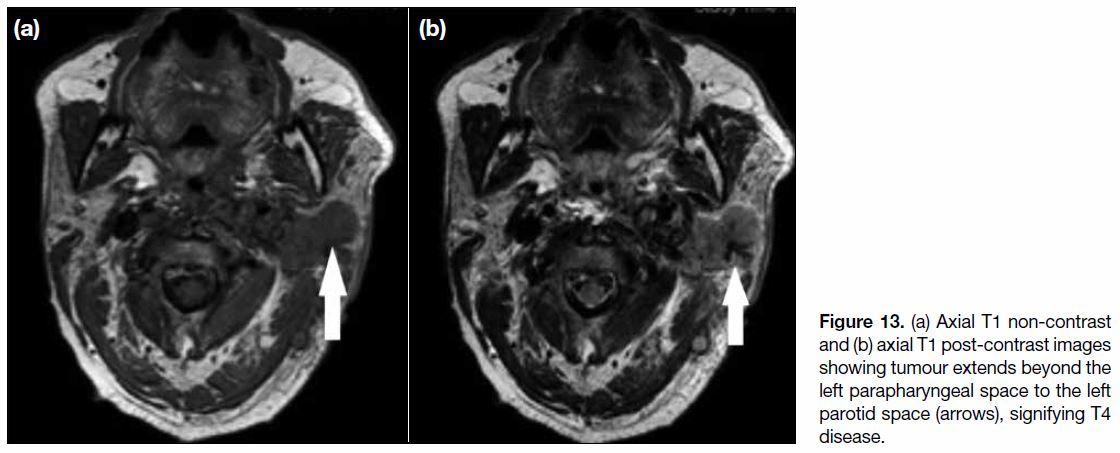
T4 Disease
T4 disease includes tumour with intracranial extension,
involvement of cranial nerves, hypopharynx, orbit,
parotid gland (Figure 13), and/or extensive soft tissue
infiltration beyond the lateral surface of the lateral
pterygoid muscle.[9] Involvement of the hypopharynx
is rare and associated with a much poorer outcome.
The disease is thus upgraded from T1 to T4 when
the tumour extends through the oropharynx to the
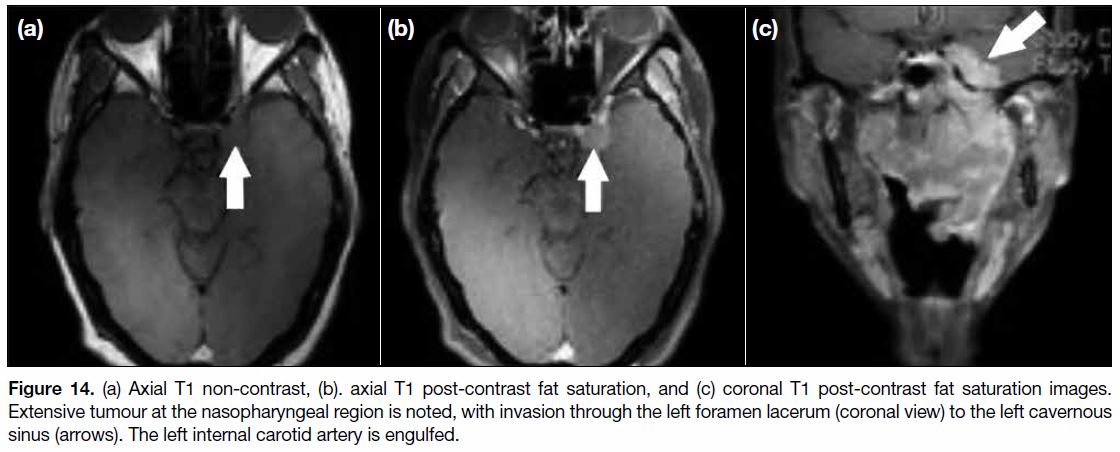
hypopharynx. When the cavernous sinus is invaded
(Figure 14), symptoms include those affected by cranial
nerve III, IV, V1, V2 and VI function. It is usually the
site of involvement when disease spread is from the
pterygopalatine fossa through the foramen rotundum
before further disease involvement of the cerebrum.
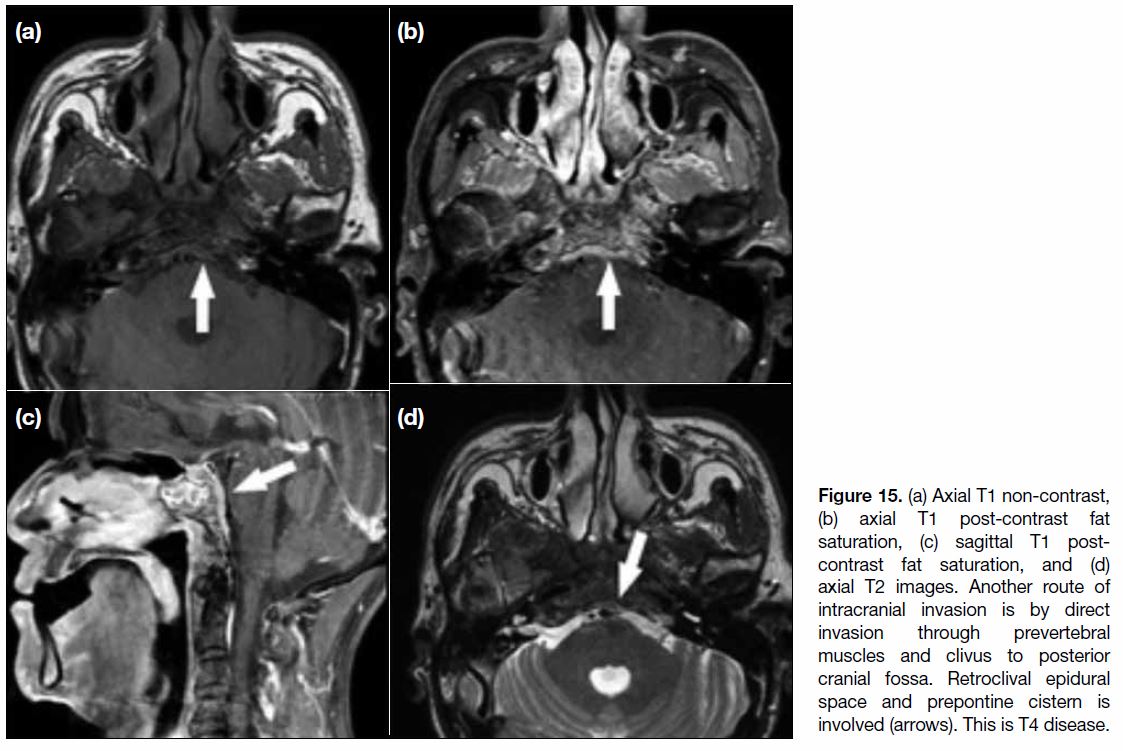
In involvement of the posterior cranial fossa, the most
common route of invasion is through the longus colli
muscle and clivus, with extension to the prepontine cistern (Figure 15).[12] In severe cases, invasion of tumour from
the pterygopalatine fossa into the orbit through the
inferior orbital fissure can be seen.[14] Proptosis may be
noted due to mass effect of the tumour.
Figure 13. (a) Axial T1 non-contrast and (b) axial T1 post-contrast images showing tumour extends beyond the left parapharyngeal space to the left parotid space (arrows), signifying T4 disease.
NX or
Figure 14. (a) Axial T1 non-contrast, (b). axial T1 post-contrast fat saturation, and (c) coronal T1 post-contrast fat saturation images.
Extensive tumour at the nasopharyngeal region is noted, with invasion through the left foramen lacerum (coronal view) to the left cavernous
sinus (arrows). The left internal carotid artery is engulfed.
Figure 15. (a) Axial T1 non-contrast, (b) axial T1 post-contrast fat saturation, (c) sagittal T1 post-contrast fat saturation, and (d) axial T2 images. Another route of intracranial invasion is by direct invasion through prevertebral muscles and clivus to posterior cranial fossa. Retroclival epidural space and prepontine cistern is involved (arrows). This is T4 disease.
In T4 disease, “extension to parotid gland and/or extensive
soft tissue infiltration beyond the lateral surface of lateral
pterygoid muscle” in the AJCC 8th edition replaces “extension to infratemporal fossa/masticator space” in
the AJCC 7th edition. Other contents such as intracranial
extension, involvement of cranial nerves, hypopharynx,
and/or orbit are unchanged.
N STAGE
Detailed descriptions of staging of regional lymph nodes
can be found in the AJCC Cancer Staging Manual.[9]
NX or N0
NX signifies that regional lymph nodes cannot be
assessed while N0 indicates that no regional lymph node
metastasis is noted.
N1 Disease
N1 denotes unilateral metastasis in cervical lymph nodes
and/or unilateral or bilateral metastasis in retropharyngeal
lymph nodes, ≤6 cm at their largest dimension, above the caudal border of the cricoid cartilage.[9] In addition
to T2 and T1 post-contrast images for primary tumour
and nodal metastasis, diffusion-weighted imaging is also
valuable for assessment of nodal metastasis.[17] The most
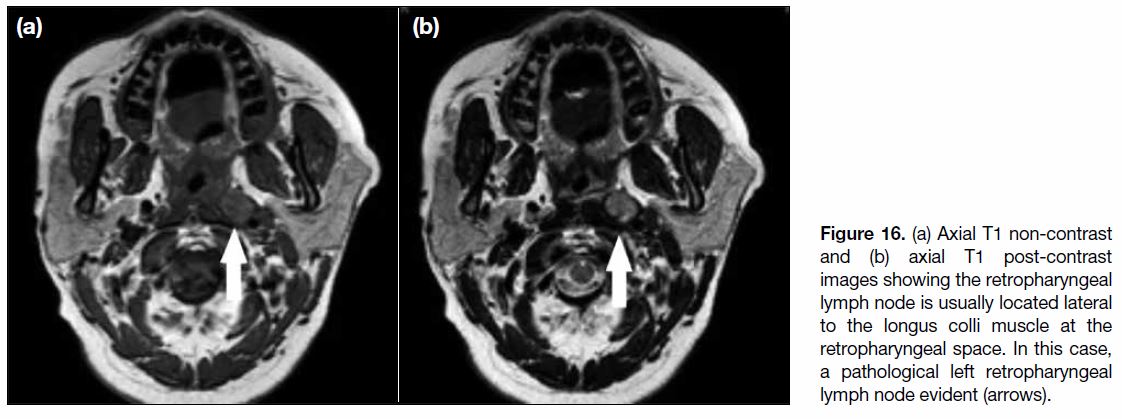
important lymph node to evaluate is the retropharyngeal
lymph node, a node located at the retropharyngeal space usually lateral to prevertebral muscle (Figure 16). It is
the most common and earliest node to be involved in
nasopharyngeal carcinoma. The lymphatic involvement
of cervical lymph nodes usually follows a specific pattern
with first involvement of the retropharyngeal lymph
nodes, then spreading from upper to lower cervical nodes.[18] Skip metastases are not common but can still
happen with cases that show involvement of cervical
nodes without involvement of the retropharyngeal lymph
node.[9] [18]
Figure 16. (a) Axial T1 non-contrast and (b) axial T1 post-contrast images showing the retropharyngeal lymph node is usually located lateral to the longus colli muscle at the retropharyngeal space. In this case, a pathological left retropharyngeal lymph node evident (arrows).
For N1 disease, “above the caudal border of cricoid
cartilage” has replaced “above supraclavicular fossa” in
the AJCC 8th edition.
N2 Disease
N2 disease is diagnosed when there are bilateral
metastases in cervical lymph nodes, ≤6 cm at the largest
dimension and above the caudal border of cricoid
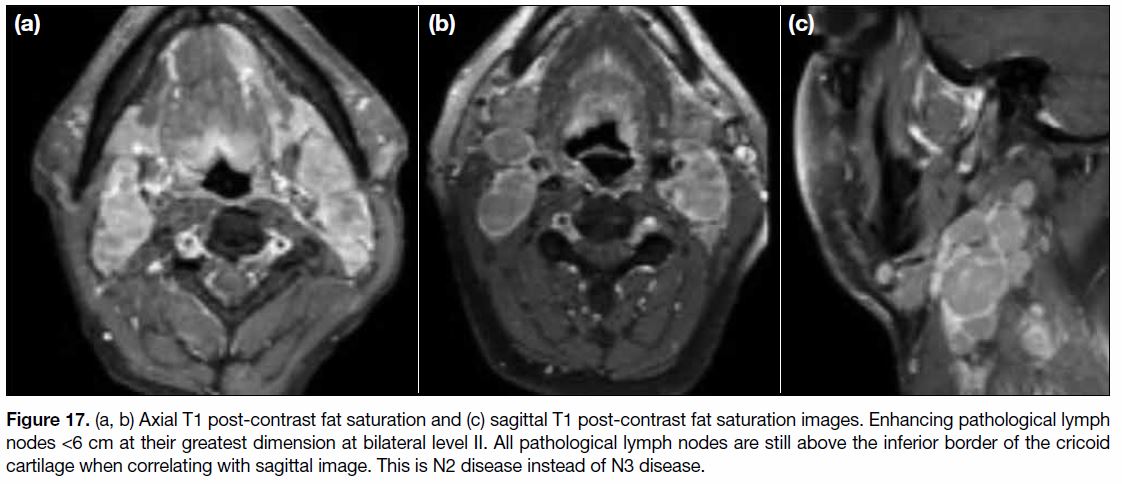
cartilage (Figure 17).[9] It denotes bilateral lymph node
involvement at level IIa, IIb, III and Va. Level IIa and IIb lymph nodes are the most commonly involved nonretropharyngeal
lymph nodes. Lymph nodes from level
IIa are the higher internal jugular group, above the
hyoid bone and in contact with the internal jugular vein.
Level IIb lymph nodes are also above the hyoid bone
but posterior to and separated from the internal jugular
vein although still anterior to the posterior border of the
sternocleidomastoid (SCM) muscle. Level III nodes are
between the level of the inferior border of the hyoid bone
and the inferior border of the cricoid cartilage and beneath
the SCM muscle. Level Va are any nodes posterior to
the posterior border of the SCM muscle and above the
inferior border of the cricoid cartilage. Involvement of
submental (level Ia) and submandibular (level Ib) lymph
nodes is uncommon but should be excluded.
Figure 17. (a, b) Axial T1 post-contrast fat saturation and (c) sagittal T1 post-contrast fat saturation images. Enhancing pathological lymph
nodes <6 cm at their greatest dimension at bilateral level II. All pathological lymph nodes are still above the inferior border of the cricoid
cartilage when correlating with sagittal image. This is N2 disease instead of N3 disease.
For N2 disease, “above the caudal border of cricoid
cartilage” has replaced “above supraclavicular fossa” in
the AJCC 8th edition.
N3 Disease
N3 disease is said to be present when any cervical lymph
nodes exceed 6 cm at their largest dimension and/or with
extension below the caudal border of the cricoid cartilage,
regardless of laterality of lymph node involvement.[9] It
refers to any pathological lymph nodes at level IV or Vb.
Involvement of these nodes carries the worst prognosis
as they are the most distant cervical lymph nodes to be
involved.[9]
For N3 disease, “below the caudal border of cricoid
cartilage” has replaced “in supraclavicular fossa” in the
AJCC 8th edition.
For AJCC criteria, the nodal staging is based on maximum
dimension, laterality, and site of lymph nodes. Any
lymph nodes along the midline are considered ipsilateral
nodes. Nonetheless identification of nodal metastases
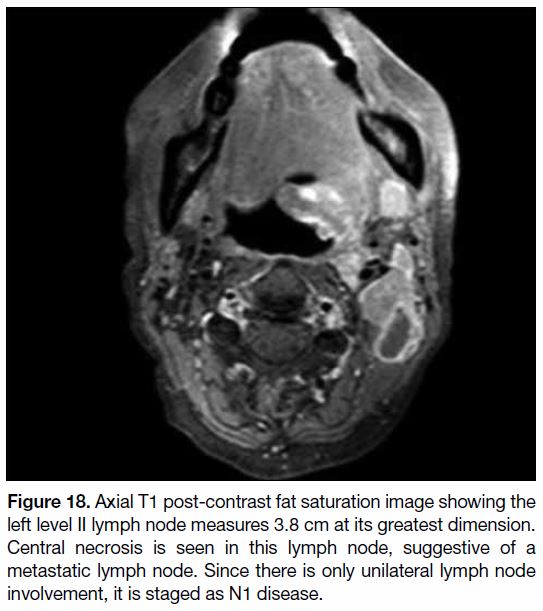
relies on a short axis of lymph nodes and morphological
features including central necrosis and extranodal
extension (Figure 18).[19] In general, most cervical lymph
nodes normally should be <10 mm in their short axis. In
particular, normal retropharyngeal lymph nodes should
not exceed 5 mm in the short axis.[20] If positron emission
tomography–computed tomography is performed, any marked hypermetabolism of lymph nodes along the
expected nodal path of disease spread should raise a
suspicion of lymph node metastases, regardless of size
of lymph nodes.[21] One more point to note is that the size
of the primary tumour does not correlate with the extent
of nodal metastases.[12]
Figure 18. Axial T1 post-contrast fat saturation image showing the
left level II lymph node measures 3.8 cm at its greatest dimension.
Central necrosis is seen in this lymph node, suggestive of a
metastatic lymph node. Since there is only unilateral lymph node
involvement, it is staged as N1 disease.
CONCLUSION
Nasopharyngeal carcinoma staging is important to guide
treatment and determine disease prognosis. Head and
neck magnetic resonance imaging adds high value in
achieving this goal. Its multiplanar capability, high soft
tissue contrast, and immense anatomical detail makes
it a powerful imaging modality. The staging method in
this article is based on AJCC 8th edition. Major changes
from the AJCC 7th edition include incorporation of
involvement of medial pterygoid, lateral pterygoid
or prevertebral muscles into T2 disease, further
specification of bony structure involvement including
skull base, cervical vertebra and pterygoid structures in
T3 disease, and discarding of terms such as infratemporal
fossa/masticator space in T4 disease and replacement
with “soft tissue infiltration beyond the lateral surface of
lateral pterygoid muscle”. Nodal staging is now classified
using the anatomical boundary of the caudal border of
the cricoid cartilage instead of supraclavicular fossa.
Continuous update on disease staging will be necessary
in the future with advancement of imaging modality and
treatment options.
REFERENCES
1. Som PM, Curtin HD, editors. Head and Neck Imaging. St. Louis (MO): Mosby-Year Book; 2003.
2. King AD, Vlantis AC, Tsang RK, Gary TM, Au AK, Chan CY, et al.
Magnetic resonance imaging for the detection of nasopharyngeal
carcinoma. AJNR Am J Neuroradiol. 2006;27:1288-91.
3. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of
nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114-9. Crossref
4. Xie SH, Yu IT, Tse LA, Mang OW, Yue L. Sex difference in the
incidence of nasopharyngeal carcinoma in Hong Kong 1983-2008:
Suggestion of a potential protective role of oestrogen. Eur J Cancer.
2013;49:150-5. Crossref
5. Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, et al.
Etiological factors of nasopharyngeal carcinoma. Oral Oncol.
2014;50:330-8. Crossref
6. Stenmark MH, McHugh JB, Schipper M, Walline HM, Komarck C,
Feng FY, et al. Nonendemic HPV-positive nasopharyngeal
carcinoma: association with poor prognosis. Int J Radiat Oncol
Biol Phys. 2014;88:580-8. Crossref
7. Yu KJ, Gao X, Chen CJ, Yang XR, Diehl SR, Goldstein A, et al.
Association of human leukocyte antigens with nasopharyngeal
carcinoma in high-risk multiplex families in Taiwan. Hum
Immunol. 2009;70:910-4. Crossref
8. Thompson LD. Update on nasopharyngeal carcinoma. Head Neck
Pathol. 2007;1:81-6. Crossref
9. Lee AW, Lydiatt WM, Colevas AD, Glastonbury CM, Le QT, O’Sullivan B, et al. Nasopharynx. In: Amin MB, editor. AJCC
Cancer Staging Manual. 8th ed. American College of Surgeons;
2018: p 103-11. Crossref
10. Lau KY, Kan WK, Sze WM, Lee AW, Chan JK, Yau TK, et al.
Magnetic resonance for T-staging of nasopharyngeal carcinoma —
the most informative pair of sequences. Jpn J Clin Oncol.
2004;34:171-5. Crossref
11. Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA,
Migliacci JC, et al. Head and Neck cancers — major changes in the
American Joint Committee on cancer eighth edition cancer staging
manual. CA Cancer J Clin. 2017;67:122-37. Crossref
12. King AD, Bhatia KS. Magnetic resonance imaging staging of
nasopharyngeal carcinoma in the head and neck. World J Radiol.
2010;2:159-65. Crossref
13. Carle LN, Ko CC, Castle JT. Nasopharyngeal carcinoma. Head Neck Pathol. 2012;6:364-8. Crossref
14. King AD, Lam WW, Leung SF, Chan YL, Teo P, Metreweli C.
MRI of local disease in nasopharyngeal carcinoma: tumour extent
vs tumour stage. Br J Radiol. 1999;72:734-41. Crossref
15. Harnsberger HR, Osborn AG. Differential diagnosis of head and
neck lesions based on their space of origin. 1. The suprahyoid part
of the neck. AJR Am J Roentgenol. 1991;157:147-54. Crossref
16. Wenig BM, Richardson M. Nasal cavity, paranasal sinuses, and
nasopharynx. In: Weidner N, Cote R, Suster S, Weiss L, editors.
Modern Surgical Pathology. 2nd ed. Philadelphia: Saunders; 2009:
p 141-207. Crossref
17. Li H, Liu XW, Geng ZJ, Wang DL, Xie CM. Diffusion-weighted
imaging to differentiate metastatic from non-metastatic
retropharyngeal lymph nodes in nasopharyngeal carcinoma.
Dentomaxillofac Radiol. 2015;44:20140126. Crossref
18. Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ. Patterns of regional
lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis
of clinical evidence. BMC Cancer. 2012;12:98. Crossref
19. Lu L, Wei X, Li YH, Li WB. Sentinel node necrosis is a negative
prognostic factor in patients with nasopharyngeal carcinoma: a
magnetic resonance imaging study of 252 patients. Curr Oncol.
2017;24:e220-5. Crossref
20. Zhang GY, Liu LZ, Wei WH, Deng YM, Li YZ, Liu XW.
Radiologic criteria of retropharyngeal lymph node metastasis
in nasopharyngeal carcinoma treated with radiation therapy.
Radiology. 2010;255:605-12. Crossref
21. Lin XP, Zhao C, Chen MY, Fan W, Zhang X, Zhi SF, et al. Role
of 18F-FDG PET/CT in diagnosis and staging of nasopharyngeal
carcinoma [in Chinese]. Ai Zheng. 2008;27:974-8.