Post-irradiation Changes and Complications of Nasopharyngeal Carcinoma: A Review of Imaging Features
PICTORIAL ESSAY
Post-irradiation Changes and Complications of Nasopharyngeal Carcinoma: A Review of Imaging Features
JCY Lau, JHM Cheng, SY Luk, JLS Khoo
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Correspondence: Dr JCY Lau, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong. Email: jackylcy135@yahoo.com.hk
Submitted: 26 Mar 2019; Accepted: 3 Jun 2019.
Contributors: JHMC, SYL and JLSK designed the study; JCYL and JHMC acquired and analysed the data; JCYL drafted the manuscript;
JHMC, SYL and JLSK critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Funding/Support: This pictorial essay received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Hospital Authority Hong Kong East Cluster Research Ethics Committee (Ref
HKECREC-2019-037).
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a relatively
common malignancy in Hong Kong. Radiotherapy
(RT) plays a major role in the treatment of early and
intermediate stage disease. Despite advancement from
conventional two-dimensional RT to three-dimensional
or intensity-modulated RT, post-irradiation changes and
complications are still commonly encountered. These
changes may evolve over time and may manifest early
or years after completion of RT. Differentiating these
expected post-irradiation changes from residual tumour
and disease recurrence can be challenging.
This article aims to review the imaging features of
common post-irradiation changes at different stages of
treatment and a broad spectrum of irradiation-related
complications in patients with NPC.
COMMON POST-IRRADIATION CHANGES
Mucocutaneous Changes
Mucocutaneous tissue is commonly affected by radiation
due to its high radiosensitivity, causing mucositis.
Inflammation can be due to direct irradiation and/or
increased epithelial susceptibility to infection. Diagnosis
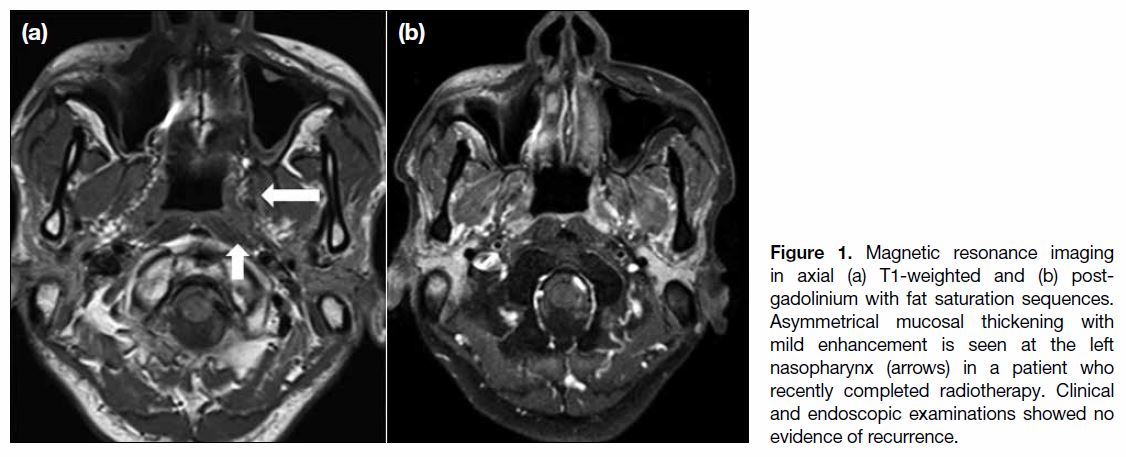
is based mainly on clinical findings, although pharyngeal mucosal hyperenhancement and thickening can be
encountered radiologically at the early stage (Figure 1).
Figure 1. Magnetic resonance imaging in axial (a) T1-weighted and (b) post-gadolinium with fat saturation sequences. Asymmetrical mucosal thickening with mild enhancement is seen at the left nasopharynx (arrows) in a patient who recently completed radiotherapy. Clinical and endoscopic examinations showed no evidence of recurrence.
Chronic mucositis can appear as mucosal atrophy,
necrosis and/or ulcerative changes, posing difficulty in
distinguishing them from malignant changes.[1] Other chronic changes including choanal atresia, paranasal sinus mucocele, and polyp formation have also been
reported.[2]
Glandular Changes
One of the most common potentially highly disabling
side-effects of RT in the head and neck region is
xerostomia.[2] The salivary glands are frequently affected
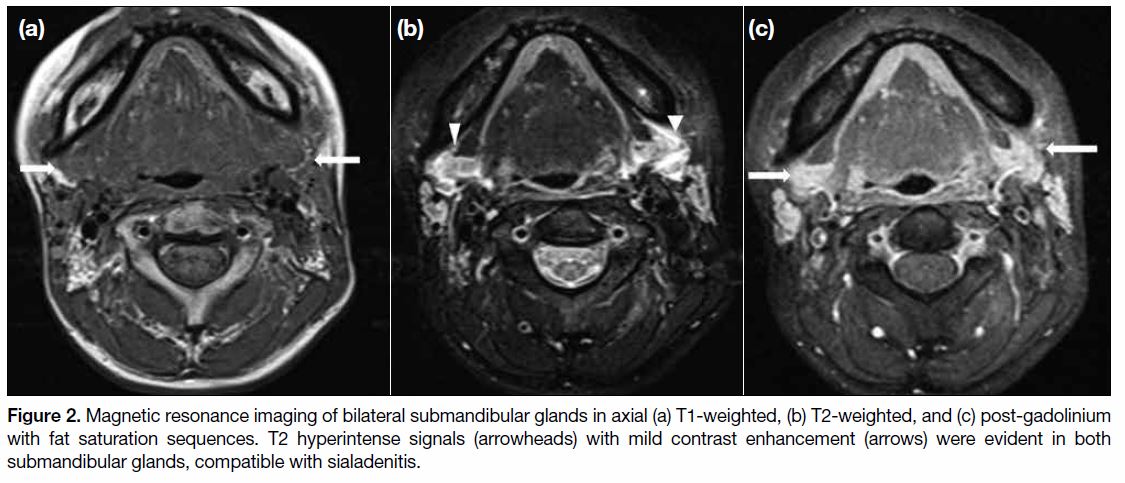
by radiation, transiently or permanently. Glandular tissue
may appear oedematous (Figure 2) with heterogeneous
signal and enhancement on magnetic resonance imaging
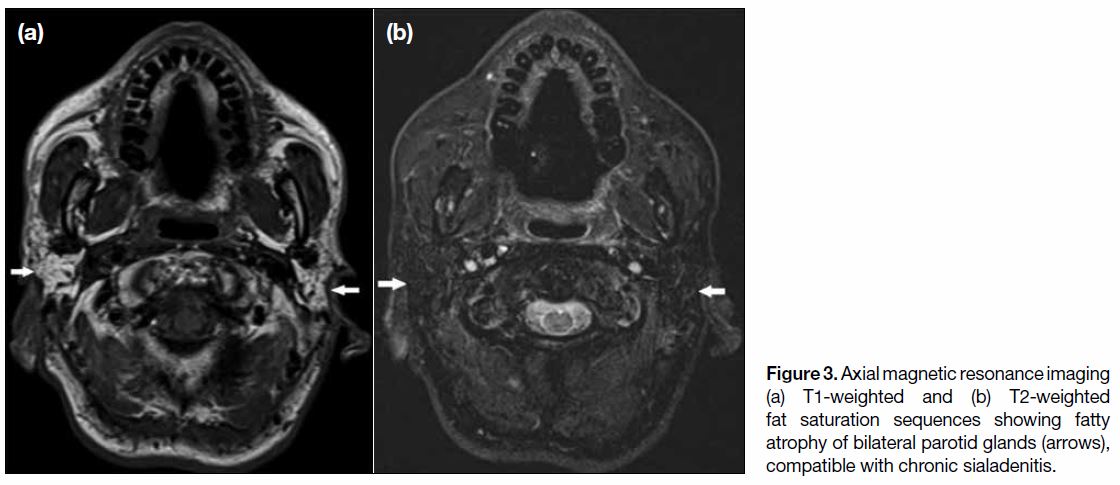
(MRI) at the early treatment stage. Atrophy and/or
fatty infiltration may be encountered at a later stage of
treatment (Figure 3).[3]
Figure 2. Magnetic resonance imaging of bilateral submandibular glands in axial (a) T1-weighted, (b) T2-weighted, and (c) post-gadolinium
with fat saturation sequences. T2 hyperintense signals (arrowheads) with mild contrast enhancement (arrows) were evident in both
submandibular glands, compatible with sialadenitis.
Figure 3. Axial magnetic resonance imaging
(a) T1-weighted and (b) T2-weighted
fat saturation sequences showing fatty
atrophy of bilateral parotid glands (arrows),
compatible with chronic sialadenitis.
The pituitary gland may also be damaged by irradiation,
impairing the hypothalamic-pituitary hormonal axis with
consequent hormonal deficiencies. Nonetheless MRI
findings are usually unremarkable despite the presence
of symptomatic neuroendocrine deficiency.[2]
Sinusitis and Otomastoiditis
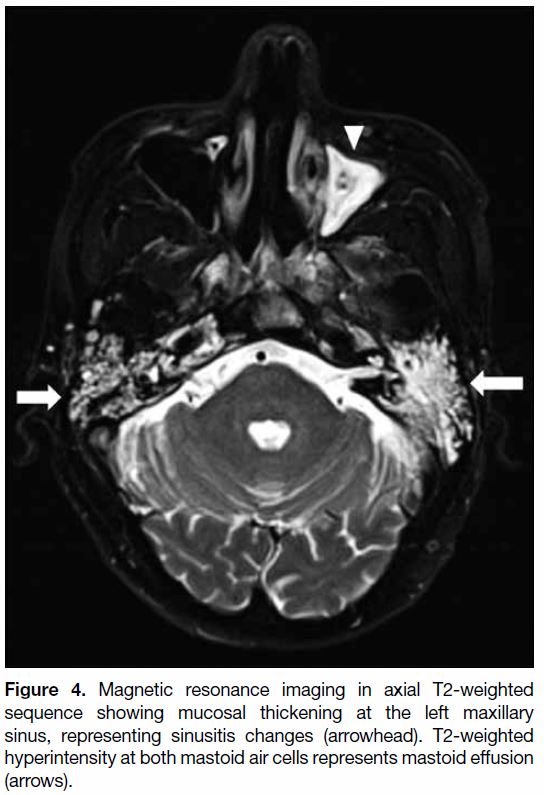
Post-irradiation and at the early stage of treatment,
patients commonly experience a certain degree of
sinusitis and/or otomastoiditis (Figure 4), mainly due to
reactive oedema of the mucosa and blockage of meatal
openings. The incidence of mastoiditis usually increases
during the first few months of treatment. Imaging features
include T2-weighted (TW2) hyperintense effusion, with
or without contrast enhancement and restricted diffusion.
In severe cases, subperiosteal abscess may develop.[4]
Figure 4. Magnetic resonance imaging in axial T2-weighted
sequence showing mucosal thickening at the left maxillary
sinus, representing sinusitis changes (arrowhead). T2-weighted
hyperintensity at both mastoid air cells represents mastoid effusion
(arrows).
Over time, chronic sinusitis and/or otomastoiditis are
observed in some irradiated patients, although the
incidence is lower than during early treatment.[4] The
acute inflammation may become crusted with adhesions
months to years after completion of RT.
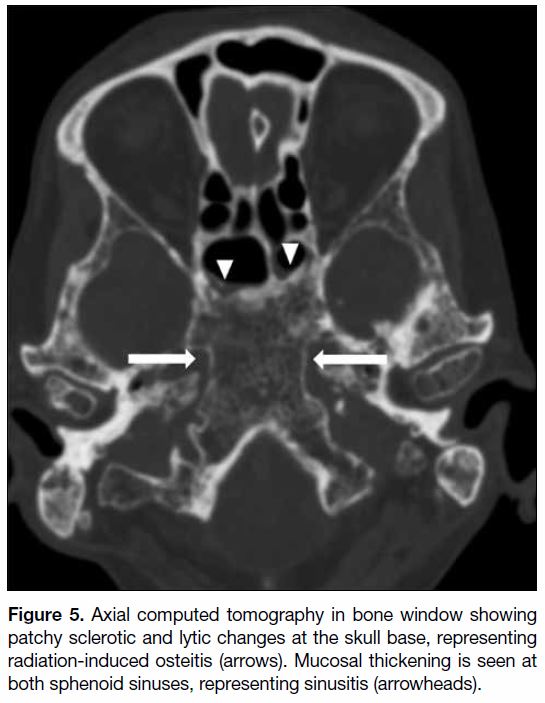
Radiation-induced osteitis
Radiation-induced osteitis is commonly asymptomatic
and involves the sphenoid bone at the skull base and upper
cervical spine. Radiation-induced fatty replacement of marrow is the most common osseous imaging
abnormality in post-irradiation patients, although initial
imaging may be normal. On computed tomography,
a mottled appearance of the skull base with mixed
patchy sclerosis and lucency and coarsened trabeculum
can be observed (Figure 5).[1] Further progression to
osteoradionecrosis with osseous changes can be seen in
some cases and will be further discussed later on.
Figure 5. Axial computed tomography in bone window showing
patchy sclerotic and lytic changes at the skull base, representing
radiation-induced osteitis (arrows). Mucosal thickening is seen at
both sphenoid sinuses, representing sinusitis (arrowheads).
Others
Other common changes including trismus, skin and neck
fibrosis are beyond the scope of this review.
POST-RADIATION COMPLICATIONS
Radiation Injury to the Nervous System
Temporal Lobe Necrosis
One of the most debilitating and serious neurological
complications is temporal lobe necrosis (TLN). It has a
latent period of 1.5 to 13 years.[5] The inferomedial aspects
of the temporal lobes are most commonly involved due
to their close proximity to the radiation field at the skull
base, with bilateral involvement in up to approximately
two-thirds of patients.[6] Histological examination shows
oedema, reactive gliosis and demyelination, followed by cavitation and necrosis. Imaging findings include focal or
extensive homogenous T2W hyperintensity in the white
matter, representing white matter rarefaction of myelin
or oedema. Associated mass effect may be present,
especially when there is extensive white matter injury.
Grey matter lesions occur in up to 90% of patients but
are usually less extensive,[7] with the inferior aspects of the
temporal lobes being most susceptible since they receive
a higher radiation dosage. MRI shows necrotic foci and
contrast enhancing lesions that may progress or resolve
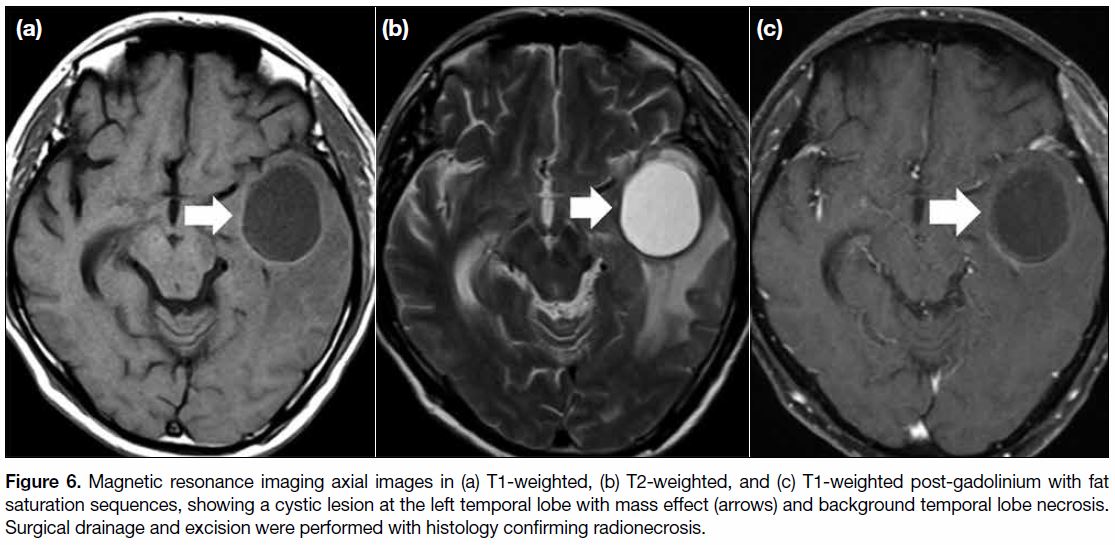
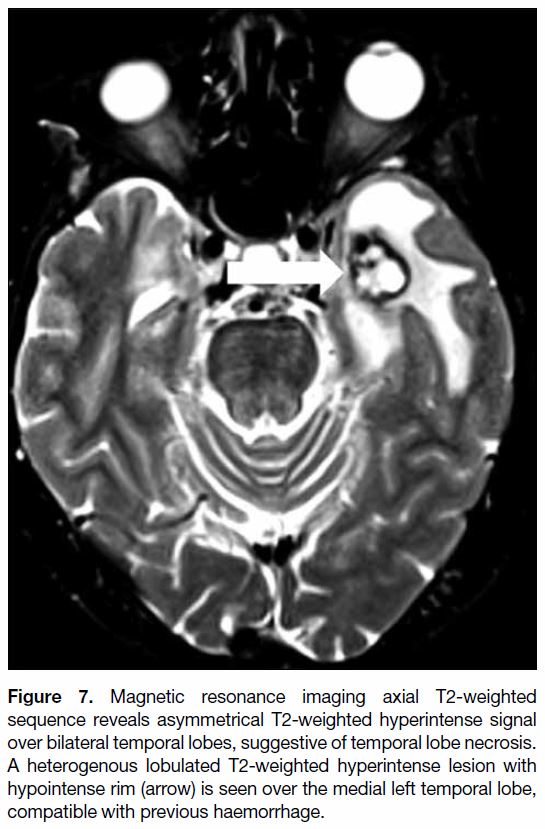
over time.[2] In some cases, TLN may be complicated by
cystic formation (Figure 6) or haemorrhage at later stages
(Figure 7).[8] Steroid is thought to be useful to reduce the oedema of TLN.[7]
Figure 6. Magnetic resonance imaging axial images in (a) T1-weighted, (b) T2-weighted, and (c) T1-weighted post-gadolinium with fat
saturation sequences, showing a cystic lesion at the left temporal lobe with mass effect (arrows) and background temporal lobe necrosis.
Surgical drainage and excision were performed with histology confirming radionecrosis.
Figure 7. Magnetic resonance imaging axial T2-weighted
sequence reveals asymmetrical T2-weighted hyperintense signal
over bilateral temporal lobes, suggestive of temporal lobe necrosis.
A heterogenous lobulated T2-weighted hyperintense lesion with
hypointense rim (arrow) is seen over the medial left temporal lobe,
compatible with previous haemorrhage.
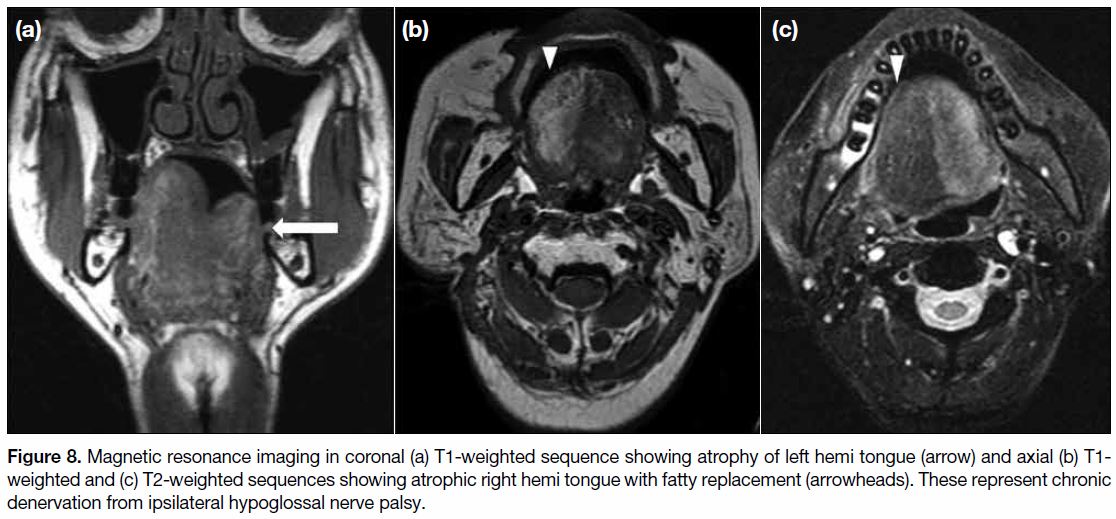
Cranial Nerve Palsy
The second most common site of neurological injury
is the cranial nerves, especially the hypoglossal nerve,
followed by the vagus nerve and recurrent laryngeal
nerve.[9] MRI may demonstrate secondary signs of cranial
nerve palsy that include oedema, fatty infiltration and
atrophy of the respective muscles supplied by the affected
cranial nerve, namely the ipsilateral hemitongue in
hypoglossal nerve palsy (Figure 8), sternocleidomastoid
and trapezius muscles in spinal accessory nerve palsy
(Figure 9).
Figure 8. Magnetic resonance imaging in coronal (a) T1-weighted sequence showing atrophy of left hemi tongue (arrow) and axial (b) T1-weighted and (c) T2-weighted sequences showing atrophic right hemi tongue with fatty replacement (arrowheads). These represent chronic
denervation from ipsilateral hypoglossal nerve palsy.
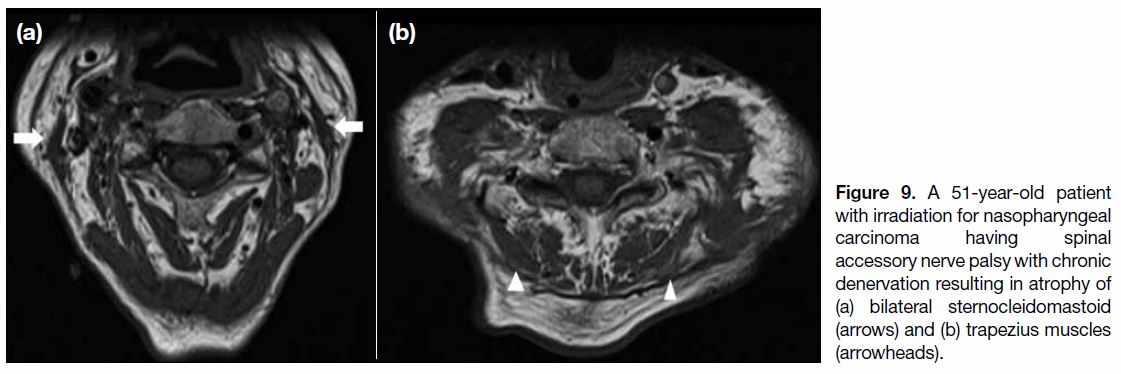
Figure 9. A 51-year-old patient
with irradiation for nasopharyngeal carcinoma having spinal accessory nerve palsy with chronic denervation resulting in atrophy of (a) bilateral sternocleidomastoid (arrows) and (b) trapezius muscles (arrowheads).
Brachial Plexopathy
Although injury to the nervous system at the lower neck
is less commonly seen nowadays, brachial plexopathy
may be encountered occasionally. On MRI, the brachial
plexus may appear thickened with T2W hyperintensity
and contrast enhancement (Figure 10).[10] Atrophy of the
serratus anterior, rotator cuff muscles can be seen in
chronic denervation. It has a peak incidence 10 to 20
months post-radiation.
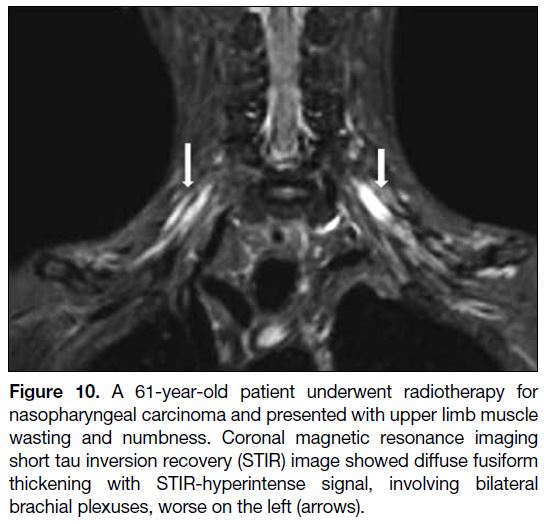
Figure 10. A 61-year-old patient underwent radiotherapy for
nasopharyngeal carcinoma and presented with upper limb muscle
wasting and numbness. Coronal magnetic resonance imaging
short tau inversion recovery (STIR) image showed diffuse fusiform
thickening with STIR-hyperintense signal, involving bilateral
brachial plexuses, worse on the left (arrows).
Cerebral Abscess
Cerebral abscess formation may also be encountered,
with postulated ascending infection through bony defects
from radiation-induced osteitis, sinusitis or mastoiditis. Irregular rim enhancement can be seen on MRI (Figure 11). It usually demonstrates less restricted diffusion
(Figure 12).[2]
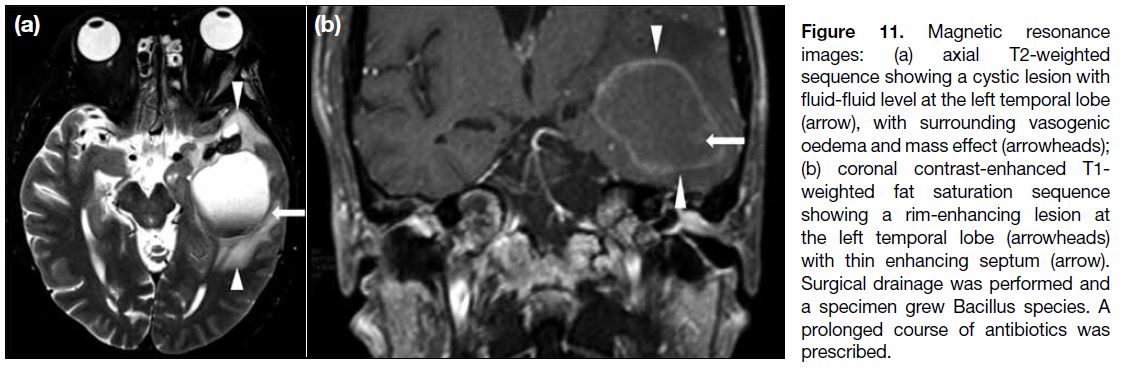
Figure 11. Magnetic resonance
images: (a) axial T2-weighted sequence showing a cystic lesion with fluid-fluid level at the left temporal lobe (arrow), with surrounding vasogenic oedema and mass effect (arrowheads); (b) coronal contrast-enhanced T1-weighted fat saturation sequence showing a rim-enhancing lesion at the left temporal lobe (arrowheads) with thin enhancing septum (arrow). Surgical drainage was performed and a specimen grew Bacillus species. A prolonged course of antibiotics was prescribed.
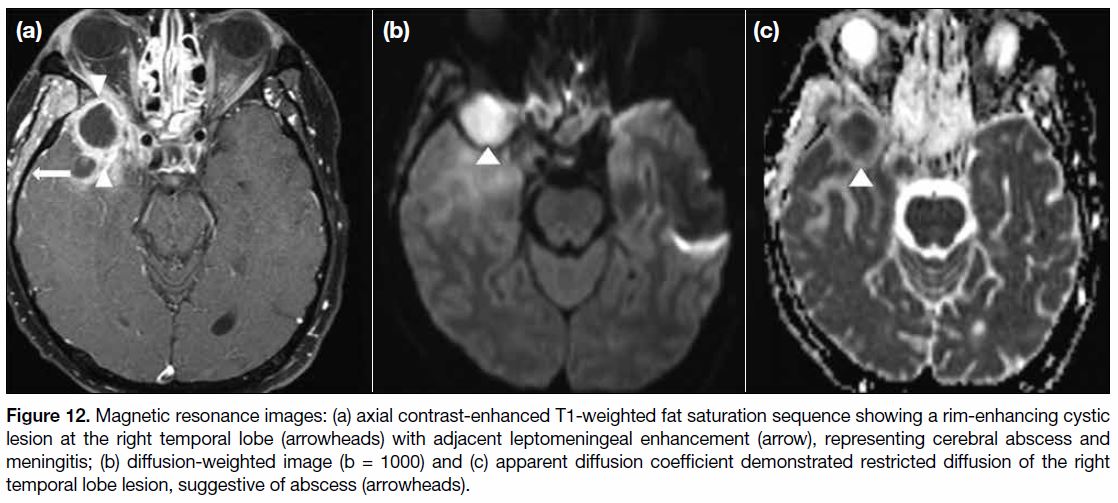
Figure 12. Magnetic resonance images: (a) axial contrast-enhanced T1-weighted fat saturation sequence showing a rim-enhancing cystic
lesion at the right temporal lobe (arrowheads) with adjacent leptomeningeal enhancement (arrow), representing cerebral abscess and
meningitis; (b) diffusion-weighted image (b = 1000) and (c) apparent diffusion coefficient demonstrated restricted diffusion of the right
temporal lobe lesion, suggestive of abscess (arrowheads).
Radiation-induced Osteoradionecrosis
Osteoradionecrosis (ORN) occurs when radiation
fibrosis, bony destruction and vascular injury result in
tissue breakdown, with the risk highest during the first
6 to 12 months following RT.[2] These result in bony
necrosis and sequestration. Computed tomography
detects cortical disruption and loss of marrow
trabeculations while MRI shows heterogeneous T1-weighted low-to-intermediate and T2W intermediate-to-high
marrow signal (Figure 13). Surrounding soft tissue mass may be found and can mimic tumour recurrence
or superimposed osteomyelitis, rendering radiological
diagnosis challenging.[2] A constellation of clinical
findings together with close monitoring and radiological
follow-up are helpful and essential in these cases.
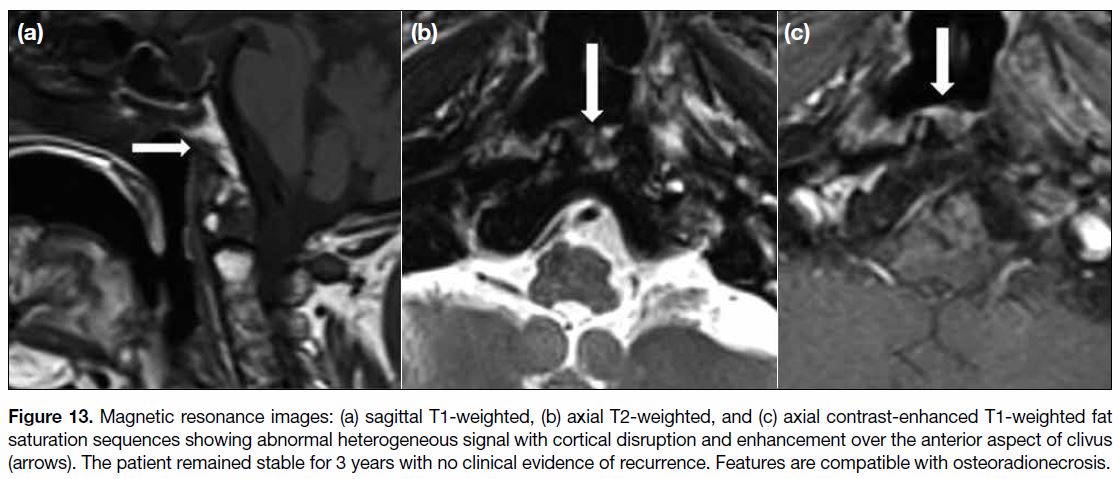
Figure 13. Magnetic resonance images: (a) sagittal T1-weighted, (b) axial T2-weighted, and (c) axial contrast-enhanced T1-weighted fat
saturation sequences showing abnormal heterogeneous signal with cortical disruption and enhancement over the anterior aspect of clivus
(arrows). The patient remained stable for 3 years with no clinical evidence of recurrence. Features are compatible with osteoradionecrosis.
Common sites of ORN include skull base and mandible,
rarely the cervical spine.[11] With disrupted bony cortex
of ORN tissue, microbes can ascend to the cranial fossa
from paranasal sinuses or the otomastoid system with
pneumocephaly. ORN of the mandible can allow spread
of dental infections.
For ORN of the mandible, a staging system based on
the symptoms, clinical and radiographical findings have
been adopted to facilitate patient management. Presence
of bony destruction indicates more advanced disease and need for more aggressive treatment. Dependent on disease
stage, treatment ranges from conservative management
with antibiotics and mouthwashes to hyperbaric oxygen
therapy and ultrasound therapy or surgery.[12]
Radiation-induced Neoplasms
To diagnose a radiation-induced neoplasm, one must
occur after a sufficient latency period and histologically
differ to the primary tumour.[1] Radiation-induced
neoplasm is rare. Common histological types are
radiation-induced sarcomas (RIS) and squamous cell
carcinomas (SCC).[13] RIS usually occur 5 to 10 years
after radiation, arising in high-dose field zones such
as the maxilla and skull base.[5] Various histological
subtypes are observed in RIS, including osteosarcoma
and malignant fibrous histiocytoma. Imaging features
can be variable but typically include a rapidly growing
destructive mass with heterogeneous signals, with or
without calcification[2] (Figures 14 and 15).
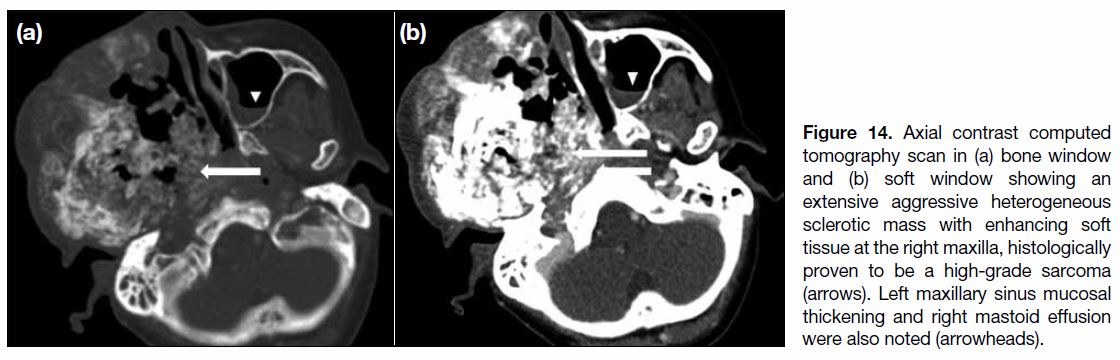
Figure 14. Axial contrast computed tomography scan in (a) bone window and (b) soft window showing an extensive aggressive heterogeneous sclerotic mass with enhancing soft tissue at the right maxilla, histologically proven to be a high-grade sarcoma (arrows). Left maxillary sinus mucosal thickening and right mastoid effusion were also noted (arrowheads).
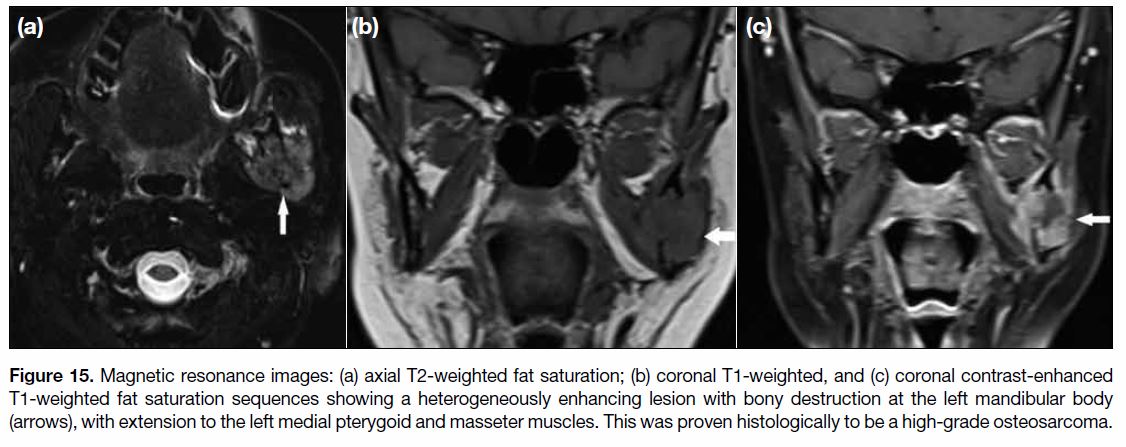
Figure 15. Magnetic resonance images: (a) axial T2-weighted fat saturation; (b) coronal T1-weighted, and (c) coronal contrast-enhanced
T1-weighted fat saturation sequences showing a heterogeneously enhancing lesion with bony destruction at the left mandibular body
(arrows), with extension to the left medial pterygoid and masseter muscles. This was proven histologically to be a high-grade osteosarcoma.
SCC typically occur at the temporal bone and external
auditory canals and are seen 10 to 15 years after
irradiation.[3] Like RIS, SCC also carry a poor prognosis[14]
and surgery is the only chance of cure, provided the
tumour is detected at an early stage.
Radiation-induced Vascular Complications
Accelerated atherosclerosis, thrombosis, and intimal
hyperplasia can result in large vessel stenosis and/or
occlusion. Imaging findings may reveal atypically
located, diffuse and less calcified atherosclerosis.[2]
Atherosclerosis can be found at the common carotid
and internal carotid arteries, in addition to the carotid
sinus.
Rare but devastating vascular complications include
pseudoaneurysm formation, (Figure 16) carotid
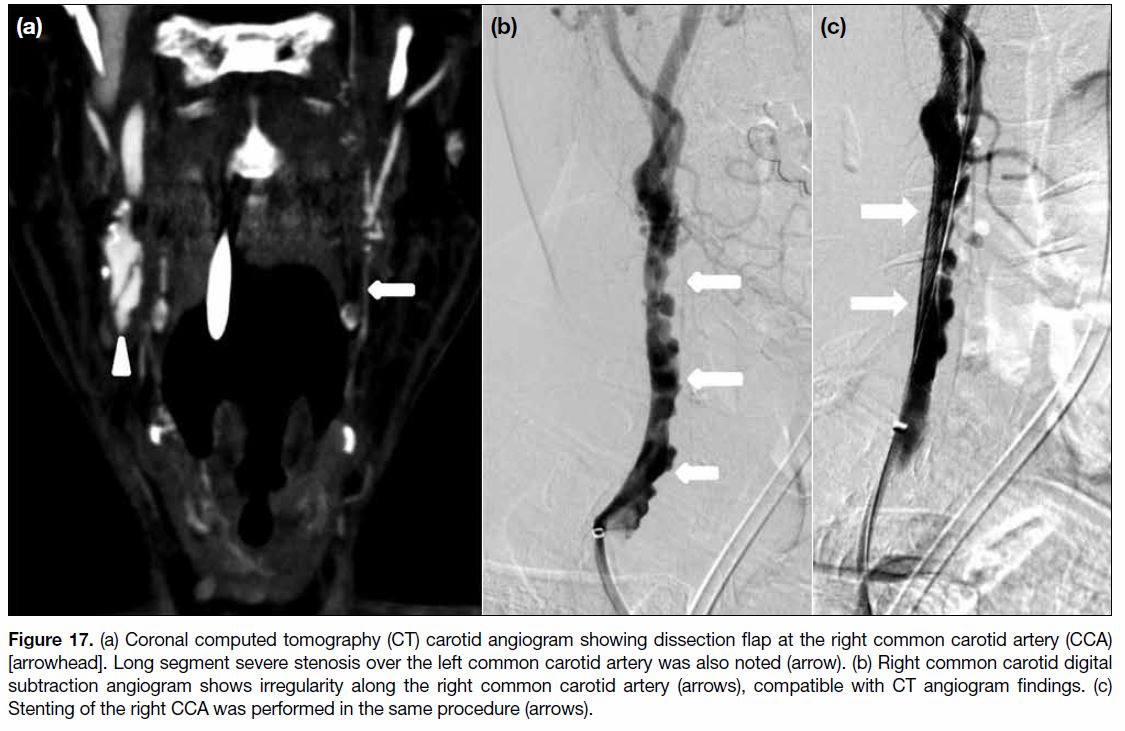
stenosis or dissection (Figure 17). Therapeutic vascular
intervention may be required and includes stenting for stenosis or dissection and coil embolisation for
pseudoanerysm.[15]
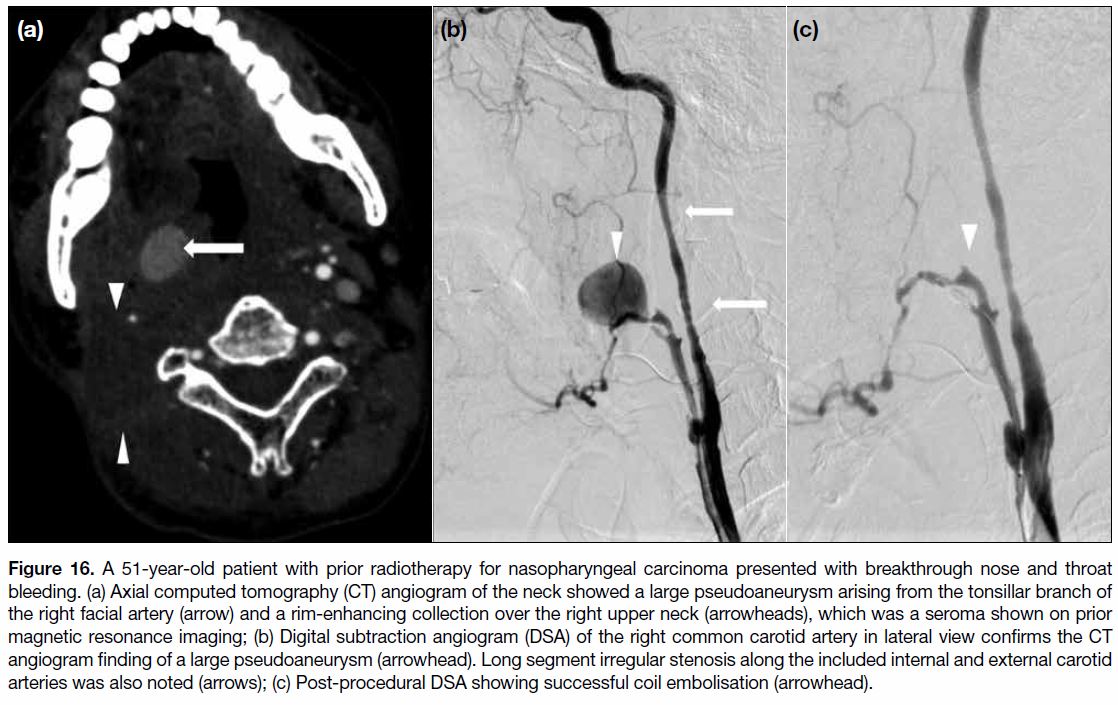
Figure 16. A 51-year-old patient with prior radiotherapy for nasopharyngeal carcinoma presented with breakthrough nose and throat
bleeding. (a) Axial computed tomography (CT) angiogram of the neck showed a large pseudoaneurysm arising from the tonsillar branch of
the right facial artery (arrow) and a rim-enhancing collection over the right upper neck (arrowheads), which was a seroma shown on prior
magnetic resonance imaging; (b) Digital subtraction angiogram (DSA) of the right common carotid artery in lateral view confirms the CT
angiogram finding of a large pseudoaneurysm (arrowhead). Long segment irregular stenosis along the included internal and external carotid
arteries was also noted (arrows); (c) Post-procedural DSA showing successful coil embolisation (arrowhead).
Figure 17. (a) Coronal computed tomography (CT) carotid angiogram showing dissection flap at the right common carotid artery (CCA)
[arrowhead]. Long segment severe stenosis over the left common carotid artery was also noted (arrow). (b) Right common carotid digital
subtraction angiogram shows irregularity along the right common carotid artery (arrows), compatible with CT angiogram findings. (c)
Stenting of the right CCA was performed in the same procedure (arrows).
CONCLUSION
RT, which plays a major role in the treatment of NPC,
can cause a wide spectrum of expected post-procedure changes and complications in the head and neck region.
Familiarisation with the relevant imaging features is
essential in post-irradiation imaging surveillance to
ensure early diagnosis to guide subsequent management.
Imaging features atypical of post-irradiation changes
should raise the suspicion of other pathologies including disease recurrence and radiation-related complications.
REFERENCES
1. Bharatha A, Yu E, Symons SP, Bartlett ES. Early and late-term
effects of radiotherapy in head and neck imaging. Can Assoc radiol
J. 2012;63:119-28. Crossref
2. King AD, Ahuja AT, Yeung DK, Wong JK, Lee YY, Lam WW, et al.
Delayed complications of radiotherapy treatment for nasopharyngeal
carcinoma: imaging findings. Clin Radiol. 2007;62:195-203. Crossref
3. Glastonbury CM, Parker EE, Hoang JK. The postradiation neck:
evaluating response to treatment and recognizing complications.
AJR Am J Roentgenol. 2010;195:W164-71. Crossref
4. Yao JJ, Zhou GQ, Xiao LY, Ling LT, Lei C, Yan PM, et al.
Incidence of and risk factors for mastoiditis after intensity
modulated radiotherapy in nasopharyngeal carcinoma. PLoS ONE.
2015;10:e0131284. Crossref
5. Abdel Razek AA, King A. MRI and CT of nasopharyngeal
carcinoma. AJR Am J Roentgenol. 2012;198:11-8. Crossref
6. Chong VF, Fan YF, Mukherji SK. Radiation-induced temporal
lobe changes: CT and MR imaging characteristics. AJR Am J
Roentgenol. 2000;175:431-6. Crossref
7. Chan YL, Leung SF, King AD, Choi PH, Metreweli C. Late
radiation injury to the temporal lobes: morphologic evaluation at
MR imaging. Radiology. 1999;213:800-7. Crossref
8. Cheng KM, Chan CM, Fu YT, Ho LC, Cheung FC, Law CK. Acute
hemorrhage in late radiation necrosis of the temporal lobe: report of
five cases and review of the literature. J Neurooncol. 2001;51:143-
50. Crossref
9. Lin YS, Jen YM, Lin JC. Radiation-related cranial nerve palsy in
patients with nasopharyngeal carcinoma. Cancer. 2002;95:404-9. Crossref
10. Rehman I, Chokshi FH, Khosa F. MR imaging of the brachial
plexus. Clin Neuroradiol. 2014;24:207-16. Crossref
11. King AD, Griffith JF, Abrigo JM, Leung SF, Yau FK, Tse GM,
et al. Osteoradionecrosis of the upper cervical spine: MR imaging
following radiotherapy for nasopharyngeal carcinoma. Eur J Radiol
2010;73:629-35. Crossref
12. Chronopoulos A, Zarra T, Ehrenfeld M, Otto S. Osteoradionecrosis
of the jaws: definition, epidemiology, staging and clinical and
radiological findings. A concise review. Int Dent J. 2018;68:22-30. Crossref
13. Abrigo JM, King AD, Leung SF, Vlantis AC, Wong JK, Tong MC,
et al. MRI of radiation-induced tumors of the head and neck in post-radiation
nasopharyngeal carcinoma. Head Neck. 2009;19:1197. Crossref
14. Chan JY, To VS, Wong ST, Wei WI. Radiation-induced
squamous cell carcinoma of the nasopharynx after radiotherapy
for nasopharyngeal carcinoma. Head Neck. 2014;36:772-5. Crossref
15. Lam JW, Chan JY, Lui WM, Ho WK, Lee R, Tsang RK.
Management of pseudoaneurysms of the internal carotid artery in
postirradiated nasopharyngeal carcinoma patients. Laryngoscope.
2014;124:2292-6. Crossref