Prostatic Arterial Embolisation in Men with Benign Prostatic Enlargement and Refractory Retention Considered High-risk Surgical Candidates
ORIGINAL ARTICLE
Prostatic Arterial Embolisation in Men with Benign Prostatic Enlargement and Refractory Retention Considered High-risk Surgical Candidates
KC Cheng1, WY Wong2, HC Chan1, KK Leung1, SM Yu1, CS Chan1, HS So1
1 Department of Surgery, United Christian Hospital, Kwun Tong, Hong Kong
2 Department of Radiology, Hong Kong Adventist Hospital, Tsuen Wan, Hong Kong
Correspondence: Dr KC Cheng, Department of Surgery, United Christian Hospital, Kwun Tong, Hong Kong. Email: bryan.ckc@gmail.com
Submitted: 29 Apr 2018; Accepted: 25 Jul 2018.
Contributors: KCC, WYW and SMY designed the study. KCC, HCC and KKL acquired the data. KCC analysed the data and drafted the
manuscript. KCC, WYW, HSS and CSC critically revised the manuscript for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Kowloon Central/Kowloon East Research Ethics Committee (Ref REC (KC/KE)-18-0142/FR-
3).
Abstract
Introduction
Prostatic arterial embolisation (PAE) is increasingly used for treatment of benign prostatic enlargement.
We investigated the use of PAE for patients with refractory urinary retention resistant to alpha blockers, who were
high-risk for conventional transurethral surgery.
Methods
This was a prospective cohort study of American Society of Anesthesiology (ASA) Class 3/4 patients.
Computed tomographic angiography of the pelvis was used to screen for feasibility of PAE. PAE was performed
using a standardised technique with tris-acryl microspheres. The primary outcome was the ability to void within
12 weeks after PAE. Secondary outcomes included complications, change in prostate size, and change in serum
prostate-specific antigen (PSA).
Results
Twenty one men were recruited. Mean (±standard deviation) age was 83±5 years, prostate size was
124± 63.4 mL, and serum PSA level was 18.4±10.5 ng/mL. Fourteen patients (66.7%) were judged to be candidates
for PAE; PAE was performed in 13 patients. Median follow-up was 10 weeks (range, 4-30 weeks). Nine (69.2%)
patients had successful voiding trials with seven of them able to void within 2 weeks after PAE. At 6 months after
PAE, PSA was decreased by 46.7%±20.9% and prostate size was reduced by 58.6%±22.5%. Larger prostate size
was significantly correlated with larger percentage reduction of prostate volume (p = 0.036). One patient developed
haematuria requiring readmission on day 5 after PAE; it resolved spontaneously.
Conclusion
PAE was effective and safe for high-risk patients with benign prostate enlargement and refractory urinary retention.
Key Words: Angiography; Embolization, therapeutic; Haematuria; Prostate-specific antigen; Prostatic hyperplasia;
Tomography, X-ray computed
中文摘要
前列腺栓塞術治療高手術風險尿瀦溜患者
鄭冠中、黃慧妍、陳開澤、梁國堅、余燊明、陳志生、蘇慶成
引言
利用前列腺栓塞術(PAE)治療良性前列腺肥大愈發普及。我們研究使用PAE,治療高手術風險而不適合傳統經尿道手術的尿瀦溜患者。
方法
這是一個前贍性研究,對象是美國麻醉學會分級為3或4病人。經過盆腔電腦斷層血管掃瞄證實PAE技術可行後,病人會接受以微球進行的血管栓塞術。首要結果是量度術後十二週內自行排尿的成功率。次要結果是量度術後的併發症,前列腺體積及血清前列腺抗原(PSA)的改變。
結果
共有21位病人參與研究。平均年齡(標準差)為83 ± 5歲,前列腺體積為124 ± 63.4 mL,PSA為18.4 ± 10.5 ng/mL。14位病人(66.7%)經掃描評估後確認為PAE技術上可行。最終13位病人接受PAE治療。隨訪中位數為10周(範圍,4-30周)。9位病人(69.2%)術後成功排尿,其中 7位術後2周已能排尿。術後6個月,PSA下降46.7% ± 20.9%,前列腺體積縮減了58.6% ± 22.5%。體積較
大的腺體術後縮減較多(p = 0.036)。一位病人術後5天出現血尿須住院觀察,其後血尿自行消退。
結論
PAE為高手術風險的前列腺肥大併發尿瀦留患者提供有效及安全的治療。
INTRODUCTION
Acute urinary retention is a well-known complication in
the development of benign prostatic enlargement (BPE).[1]
In patients that have failed voiding trials or presented
with obstructive uropathy, transurethral prostate surgery
is the standard treatment. Transurethral prostate surgery,
despite its minimally invasive nature, has associated
morbidities.[2] These are of great concern in patients with
poor general health that puts them at high surgical risk.
Prostatic arterial embolisation (PAE) is an alternative
treatment for BPE, which can result in a significant
reduction in prostate volume, improvement in flow rate
and residual urine volume, and reduction in symptom
score.[3] However, there have been limited publications
on the use of PAE in patients with refractory retention.
We investigated the use of PAE for patients who were
at high surgical risks and had refractory retention, and
studied the post-PAE voiding outcomes.
METHODS
We conducted a prospective single-arm cohort study from
April 2017 to March 2018. All emergency admissions
to urology wards were screened by urologists for study
eligibility. Principles outlined in the Declaration of
Helsinki were followed. Informed consent was obtained,
and factsheets were given to patients. Inclusion and exclusion criteria are shown in Table 1. Refractory
retention was defined as emergency admissions for
acute urinary retention, and failed voiding trials within 2
weeks after given daily doses of alpha blockers. Medical
history was evaluated and an American Society of
Anesthesiologists (ASA) score was assigned to patients.
Prostate size was measured by transrectal ultrasound by
urologists using the ellipsoid formula. Prostate size was
divided into moderate enlargement (≤80 mL) and severe
enlargement (>80 mL) to assess the effect of PAE on
these two groups.
Table 1. Inclusion and exclusion criteria.
Once patients were recruited, alpha-adrenergic blockers
were discontinued. Pre-PAE uroflowmetry was not
possible as patients had indwelling catheters. Computed
tomography angiography (CTA) of the pelvis was
performed with a 64-slice multidetector CT (General
Electric Healthcare, Milwaukee [WI], US) to screen
for vascular abnormalities that might preclude PAE
and to look for any potential variant origins of the
prostatic arteries. An injection of 120 mL of iodinated
contrast (Omnipaque 350; GE Healthcare [Shanghai]
Co., Ltd. Shanghai, China) at 3.5 to 4 mL/s, depending
on the size of the angiocatheter, was administered. A
pre-contrast scan of the pelvis preceded the contrast-enhanced
acquisition. A sublingual vasodilator (0.5 mg
nitroglycerine) was given before imaging of the arterial phase to help identify small arteries. Arterial phase
imaging was then performed with bolus triggering in the
abdominal aorta plus a 10 s delay. Afterwards, the venous
phase was acquired with a mean time of 70 to 80 s from
the time of injection. A designated radiologist with 5
years of endovascular intervention experience reviewed
the CTA images and decided upon the feasibility of
PAE. If there was any doubt about the feasibility, the
first radiologist would seek a second opinion from a
radiologist with 30 years’ experience. Catheterised urine
was saved for culture and indwelling urinary catheters
were not removed until PAE.
PAE was performed as an inpatient procedure with
patients admitted one day prior to the procedure. Baseline
pre-PAE blood tests included serum prostate-specific
antigen (PSA). All antiplatelet and anticoagulation
medications were withheld perioperatively according
to physicians’ advice. Patients fasted for 6 hours before
PAE. Premedication including oral diclofenac extended-release
tablet 100 mg, oral pantoprazole 40 mg, and a
bisacodyl 10 mg suppository, administered on the day
before embolisation. Intravenous levofloxacin 500 mg
was used for antibiotic prophylaxis on-call to the
radiology suite.
PAE was performed with a standardised technique for
all recruited patients. All procedures were performed
by the two interventional radiologists. A Siemens Artis
Zee ceiling-mounted fluoroscopy unit (Siemens Medical
Solutions, Forchheim, Germany) was used for all
procedures. The procedures were performed under local
anaesthesia using 5 mL 1% lidocaine. The right femoral artery was cannulated with a 5-Fr vascular sheath.
Bilateral internal iliac angiograms were performed
using tube rotation at ipsilateral 35° and craniocaudal
angulation at 10°. The choice of angiographic
catheters for internal iliac artery angiograms (4-Fr
Cobra 1, Cordis, Bridgewater [NJ], US; 4-Fr Simmons
Sidewinder 1 Terumo, Inc., Tokyo, Japan; 5-Fr RIM,
Cook, Bloomington [IN], US) depended on the CTA
findings and operators’ preference. In case of difficult
anatomy making cannulation of the right prostatic artery
impossible via a right femoral approach, a left femoral
approach was used.
Using the CTA for reference, the arterial supply to the
prostate was identified and one or both prostatic arteries
were cannulated using a microcatheter (Merit Maestro
2.4 Fr; Merit Medical Systems Inc. South Jordan [UT],
US) with or without the use of a microguidewire. Either
a Tenor steerable guidewire 0.014 in (Merit Medical)
or a Transcend 0.014 in steerable guidewire (Boston
Scientific, Inc., Natick [MA], US) were used. When the
position of a catheter was in doubt, cone beam CT was
used for further delineation.
After successful cannulation of one or both prostatic
arteries, selective angiograms were performed before
embolisation to ensure the position of microcatheter
inside the prostatic artery, to confirm the arterial supply
to that side of the prostate, and to exclude other arterial
supply from the prostatic artery to areas other than the
prostate. Embolisation was performed using tris-acryl
microspheres (Embosphere microspheres, Merit
Medical) of diameter 300 to 500 μm. Nitroglycerine 100 μg was injected intra-arterially before embolisation
to prevent vasospasm, facilitate delivery of particles,
and minimise reflux of particles and non-target
embolisation. In order to avoid non-target embolisation,
the Embosphere particles (2 mL) were suspended in a
22-mL solution (a mixture of 8 mL normal saline and
14 mL Omnipaque 350), which was slowly injected
using a 2-mL syringe under fluoroscopic guidance
until there was contrast stasis in the prostatic artery.
Angiograms were performed after PAE to confirm
obliteration of the prostatic arteries. Technical success
was defined as successful embolisation of one or both
prostatic arteries.
After PAE, patients were kept on complete bed rest for
24 h. Any complications were managed and recorded
according to the Society of Interventional Radiology
Classification System. Diclofenac extended-release
tablets, pantoprazole, and levofloxacin were continued
for 1 week following the procedure. Patients were
discharged with an indwelling urinary catheter.
Outpatient voiding trials were scheduled for 1, 2, 4, and
12 weeks after PAE. Clinical follow-up was arranged
3 months after PAE. Clinical assessment of lower urinary
tract symptoms, uroflowmetry, post-void residual urine
volume, serum PSA, transrectal ultrasound scans, and
validated questionnaires with International Prostate
Symptoms Score and Quality of Life (IPSS/Qol) was
conducted.
Primary outcome measure was successful voiding within
12 weeks after PAE. Successful voiding was defined as
a residual <350 mL and no evidence of painful retention.
Other outcomes included complications of PAE, reduction
in prostate size, and changes in serum PSA level.
Statistical calculations were conducted using SPSS
(Windows version 22.0; IBM Corp, Armonk [NY], US).
Descriptive statistics was used to calculate the primary
and secondary outcomes. Serum PSA levels and prostate
volumes before and after PAE were compared with the
Wilcoxon signed rank test. Correlation between prostate
size and prostate size percentage reduction were tested
with Spearman’s correlation. The Mann-Whitney U
test was performed to test any difference in percentage
reduction in prostate volume between moderately and
severely enlarged glands. Statistical significance was
taken as p < 0.05.
The STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) guidelines were used in reporting of the study results.[4]
RESULTS
Twenty-one men were recruited into the study. The mean
(±standard deviation) age was 83±5 years. Eighteen
patients belonged to ASA class 3 (attributable to chronic
obstructive pulmonary disease [n = 5], congestive heart
failure [n = 3], coronary artery disease [n = 5], and poorly
controlled hypertension [n = 5]). Four patients belonged
to ASA class 4 (attributable to chronic obstructive
pulmonary disease [n = 2] and congestive heart failure
[n = 2]). The mean PSA was 18.4±10.5 ng/mL and
the mean prostate size was 124±63.4 mL. Fourteen
(66.7%) patients were deemed feasible for PAE after
CTA assessment and seven patients were excluded after
CTA (severe atherosclerosis [n = 4], small prostate size
[n = 2], metastatic prostate cancer [n = 1]) [Table 2].
Table 2. Patient demographics and CTA findings (n = 21)
One patient died of pneumonia before PAE. Thirteen
patients proceeded to PAE with 16 total embolisations.
The technical success rate was 100%. Bilateral
embolisations were successful in nine patients (Figures 1 2 3 4). Three patients had only unilateral embolisation
performed on the first attempt due to prolonged procedure
time reaching the peak dose of contrast. Embolisations of
the contralateral prostatic artery were performed in these
cases 2 months after the first embolisations. One patient
had one-sided PAE performed only, as the preprocedural
angiogram showed the entire prostate to be supplied
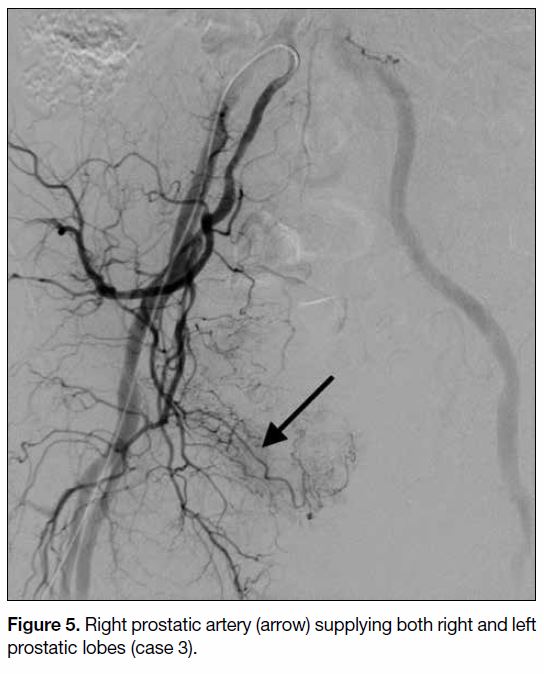
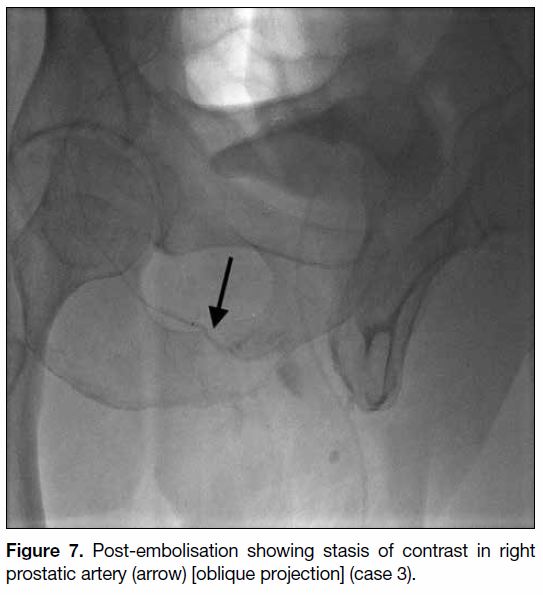
by the right prostatic artery (Figures 5 6 7). The mean
dose area product was 14,489.5 μGy·m2 (range, 5230.2-
24 212.3 μGy·m2). All 13 patients attended follow-up
and were analysed.
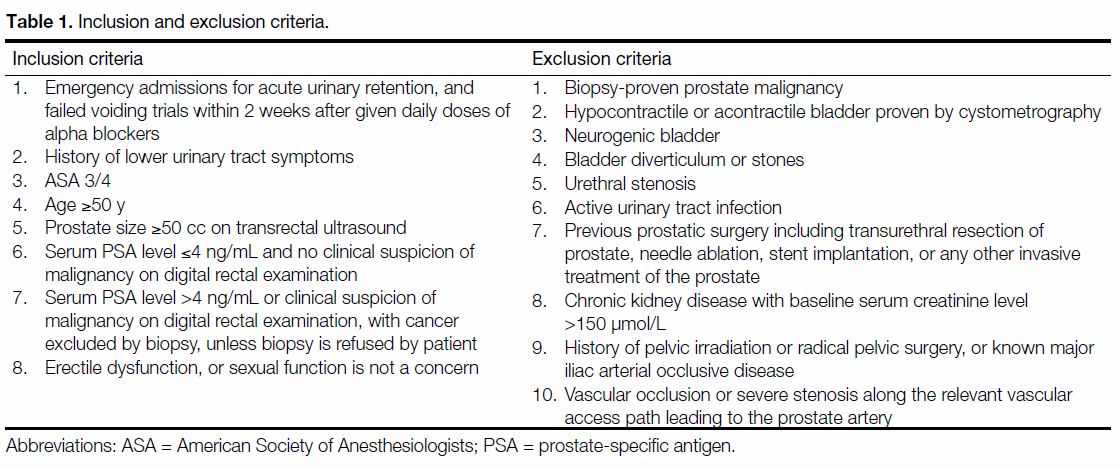
Figure 1. Right prostatic artery (arrow) before embolisation (case 1).
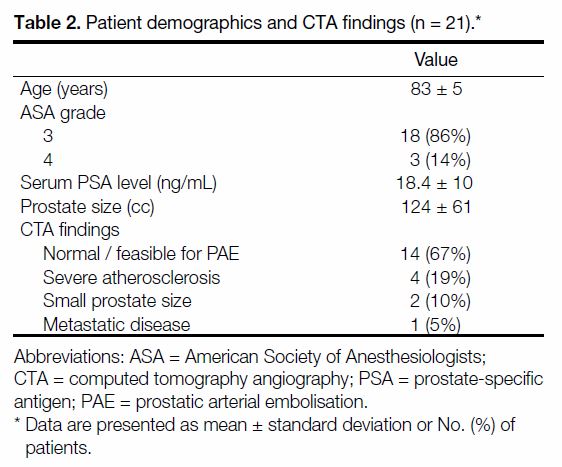
Figure 2. Post-embolisation showing stasis of contrast in right prostatic artery (arrow) [case 1].
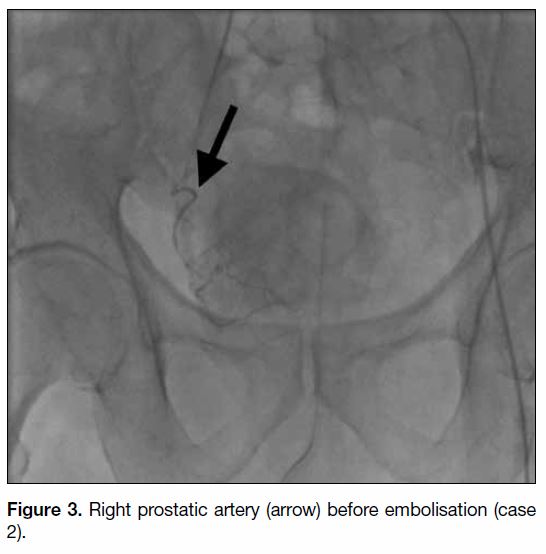
Figure 3. Right prostatic artery (arrow) before embolisation (case 2).
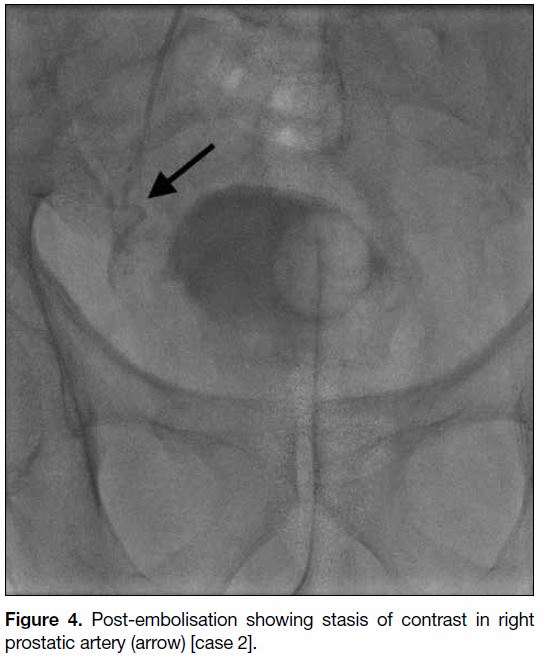
Figure 4. Post-embolisation showing stasis of contrast in right prostatic artery (arrow) [case 2].
Figure 5. Right prostatic artery (arrow) supplying both right and left prostatic lobes (case 3).
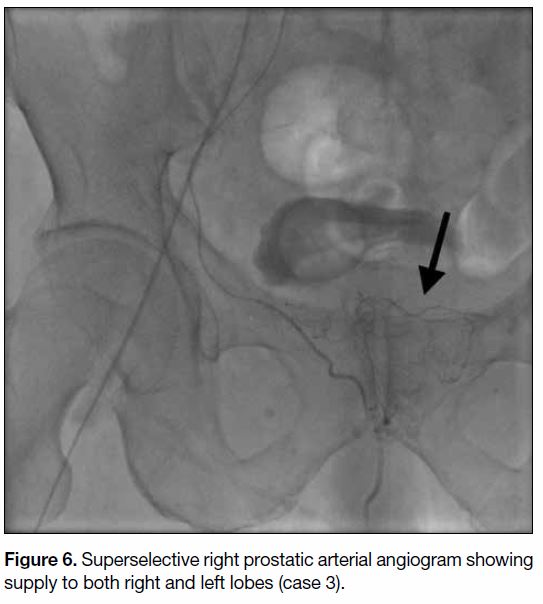
Figure 6. Superselective right prostatic arterial angiogram showing supply to both right and left lobes (case 3).
Figure 7. Post-embolisation showing stasis of contrast in right prostatic artery (arrow) [oblique projection] (case 3).
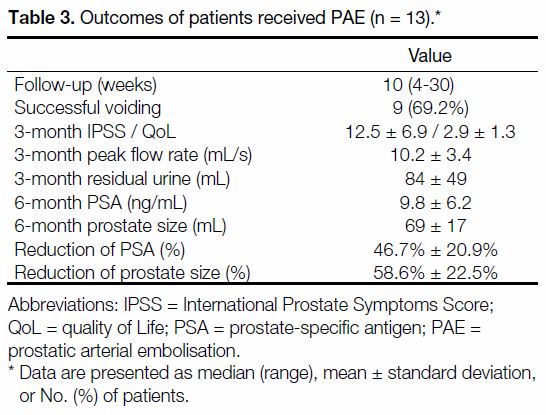
Nine patients (69.2%) had successful voiding trials with
seven of them able to void within 2 weeks after PAE
(Table 3). Despite the poor results of voiding trials, there
were reductions in PSA and prostate size. Serum PSA
levels were decreased by 46.7%±20.9% (p = 0.041) and
prostate size was reduced by 58.6%±22.5% (p = 0.028).
Larger prostate size was significantly correlated with
larger percentage reduction of prostate volume after
PAE (p = 0.036). Prostate volume percentage reduction
was 19.4% for moderately enlarged prostates and 43.6%
for severely enlarged prostates.
Table 3. Outcomes of patients received PAE (n = 13).
One patient developed haematuria requiring readmission
on day 5 after PAE. The haematuria subsided
spontaneously on observation (Society of Interventional
Radiology Classification Class B). No other adverse
events were recorded.
DISCUSSION
Refractory retention in men with evidence of BPE is
one of the indications for transurethral prostate surgery.
When successful, the surgery provides patients with the opportunity to live without an indwelling urinary
catheter. However, some men of advanced age have
co-morbidities, especially cardiorespiratory diseases,
which might preclude surgery. In these cases, long-term
indwelling urinary catheters, intermittent catheterisation,
or prostate stent insertion under local anaesthesia are
possible considerations. Prostate stent insertion is a
self-financed item in our district; thus, men with co-morbidities
and financial constraints would have to deal
with urinary catheters for the rest of their lives.
PAE is increasingly used for treatment of BPE. In the study by Pisco et al,[3] PAE was successful in 97% of
cases with a significant reduction in IPSS/QoL score and
improvement in peak flow rate. Recent meta-analyses[5] [6]
also showed a significant improvement in peak flow
rate and IPSS/QoL scores at 12 months after PAE. The
majority of published studies, however, are small and
single-site studies. The indications were heterogeneous
and few had reported the clinical success of PAE
in patients with co-morbidities.[7] [8] [9] A local study had
reported successful PAE outcomes in weaning catheters
in patients with retention of urine due to BPE.[10] In our
study we utilised PAE for high-surgical-risk patients
with indwelling catheters due to refractory retention.
The ultimate goal was to achieve spontaneous voiding
without exposure to the risk of anaesthesia and surgery.
Our pilot study showed a promising result in these
high-risk patients. The technical success rate was 100%,
although in some cases only unilateral embolisations
were possible in the first session. Conventional
angiograms had already confirmed the position of the
microcatheter and of the prostate arterial supply; thus,
cone beam CT was avoided in most cases. Nearly 70%
of patients in our cohort had successful voiding trials
after PAE, with the majority of them able to void within
2 weeks after PAE. This fast-acting clinical success of
PAE was accompanied by substantial early reduction
in prostate volume at 1 month after PAE and sustained
reduction 6 months after PAE (Figure 1). Serum PSA
levels also reduced substantially.
To the best of our knowledge, this is the first local
study to report PAE outcomes in men with refractory
retention and high surgical or anaesthetic risks. Yu et al[10]
had reported the excellent local results of PAE in
patients with complete urinary outflow obstruction.
Their group reported a higher success rate of voiding
trials (87.5% vs. 69.2%) than ours. The difference
could be explained by the younger mean age (66 vs.
83 years) and smaller prostate size (77 [study group]/
65.6 [control group] vs. 124 mL) in their cohort. The
chance of underactive bladder increased when patients
are older or when there is prolonged bladder outlet
obstruction.[11] Our lower rate of clinical success could be
related to the poorer bladder function in our older group
of patients with larger prostates. To address this issue, we
could investigate all cases with cystometrography before
and after PAE in future. Their study, however, did not
show any data on the anaesthetic risks and co-morbidities.
Our early results could potentially broaden the utilisation
of PAE in patients with high cardiorespiratory risks. More studies are needed to establish the role of PAE in
these high-risk patients and to validate our results.
The 80 mL was used in our study as a cut-off to
differentiate moderately and severely enlarged prostate
glands. This is based on the European Association
of Urology guidelines, which recommend different
treatment options for glands using the 80 mL cut-off.[12]
Conventional transurethral prostatectomy was not
recommended for prostates >80 mL, as it was considered
ineffective to resect such a large gland and possessed high
surgical risk. Instead, techniques such as holmium laser
enucleation of prostate or more invasive techniques, such
as open enucleation, were recommended.[12] However, the
clinical success of PAE had been shown to be independent
of the prostate size and even had better result in larger
prostate glands. Kurbatov et al[13] reported the efficacy
and safety of PAE for prostate volumes >80 mL. Wang
et al[14] had compared the PAE results of different prostate
sizes with cut-off of 80 mL. They found the improvement
in IPSS/QoL, peak flow rate, post-void residual, and
prostate volume reduction were all significantly better in
prostate sizes >80 mL.[14] These agree with our findings,
which showed the prostate volume reduction was much
greater in larger prostates. The reason is still uncertain.
It was hypothesised that the hypervascularity and larger
prostate size in large glands might facilitate embolisation
and result in a larger volume of infarction.[14] It makes
PAE even more appealing to those high-surgical-risk
patients with large prostates, as they could avoid more
invasive procedures such as open enucleation.
PAE was in general well tolerated and the majority
of studies reported few minor complications.[3] [5]] [10]
Commonly reported complications were urinary tract
infections (2%-19%), transient haematuria (10%),
transient haemospermia (7%), inguinal haematoma
(7%), urinary retention (8%-26%), and pelvic pain
(2%-9%).[3] [5] [15] In our cohort, only one patient had
haematuria requiring readmission, which resolved
spontaneously.
Our results were limited by the single-centre design with
small sample size. Moreover, approximately one-third
of patients were excluded owing to unfavourable
vascular anatomy or diffuse atherosclerotic disease.
These exclusions were higher than previously reported
(inclusion rate, 89.9%-100%).[3] [10] [15] Compared with other
studies, our patients were much older, with multiple
co-morbidities. After considering technical factors and
anatomical difficulties, more patients were inevitably excluded due to poor vascular status. Three patients had
unilateral PAE performed only in the first embolisation
attempt. These could be explained by the learning
curve of radiologists, as all these cases were done in
the early phase of the study. With more experiences in
catheterisation of the prostatic artery and in interpreting
the CTA, bilateral embolisations were achievable in
single session in later cases. Due to limited resources,
magnetic resonance imaging was not performed to assess
the percentage of prostate infarction. We were also unable
to show the changes in urodynamics and IPSS/QoL
results as all patients were on indwelling catheters before
PAE.
CONCLUSION
PAE was an efficacious and safe option for high-surgical-risk patients with benign prostate enlargement in
refractory retention. With this encouraging preliminary
result, we could further develop and establish the role of
PAE among the different treatment modalities for BPE
in our centre.
REFERENCES
1. Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T,
Guess HA, et al. Treatment for benign prostatic hyperplasia among
community dwelling men: the Olmsted County study of urinary
symptoms and health status. J Urol. 1999;162:1301-6. Crossref
2. Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Giling P, Gratzke C,
et al. A systematic review and meta-analysis of functional outcomes
and complications following transurethral procedures for lower
urinary tract symptoms resulting from benign prostatic obstruction:
an update. Eur Urol. 2015;67:1066-96. Crossref
3. Pisco J, Campos Pinheiro L, Bilhim T, Duarte M, Rio Tinto H,
Fernandes L, et al. Prostatic arterial embolization for benign
prostatic hyperplasia: short- and intermediate-term results.
Radiology. 2013;266:668-77. Crossref
4. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC,
Vandenbroucke JP; STROBE Initiative. The Strengthening the
Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin
Epidemiol. 2008;61:344-9. Crossref
5. Uflacker A, Haskal ZJ, Bilhim T, Patrie J, Huber T, Pisco JM.
Meta-analysis of prostatic artery embolization for benign prostatic
hyperplasia. J Vasc Interv Radiol. 2016;27:1686-97.e8. Crossref
6. Wang XY, Zong HT, Zhang Y. Efficacy and safety of prostate artery
embolization on lower urinary tract symptoms related to benign
prostatic hyperplasia: a systematic review and meta-analysis. Clin
Interv Aging. 2016;11:1609-22. Crossref
7. Bhatia S, Sinha VK, Kava BR, Gomez C, Harward S, Punnen S, et al.
Efficacy of prostatic artery embolization for catheter-dependent
patients with large prostate sizes and high comorbidity scores. J
Vasc Interv Radiol. 2018;29:78-84.e1. Crossref
8. Gabr AH, Gabr MF, Elmohamady BN, Ahmed AF. Prostatic artery
embolization: a promising technique in the treatment of high-risk
patients with benign prostatic hyperplasia. Urol Int. 2016;97:320-4. Crossref
9. Yan WH, Zhang C, Al GP, Shu Y. Prostatic arterial embolization for
benign prostatic hyperplasia in high-risk aged males [in Chinese].
Zhonghua Nan Ke Xue. 2015;21:900-3.
10. Yu SC, Cho CC, Hung EH, Chiu PK, Yee CH, Ng CF. Prostate
artery embolization for complete urinary outflow obstruction due
to benign prostatic hypertrophy. Cardiovasc Intervent Radiol.
2017;40:33-40. Crossref
11. Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V,
et al. Detrusor underactivity and the underactive bladder: a new
clinical entity? A review of current terminology, definitions,
epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389-98. Crossref
12. Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher
S, Mamoulakis C, et al. EAU guidelines on the assessment of nonneurogenic
male lower urinary tract symptoms including benign
prostatic obstruction. Eur Urol. 2015;67:1099-109. Crossref
13. Kurbatov D, Russo GI, Lepetukhin A, Dubsky S, Sitkin I, Morgia G,
et al. Prostatic artery embolization for prostate volume greater than
80 cm3: results from a single-center prospective study. Urology.
2014;84:400-4. Crossref
14. Wang M, Guo L, Duan F, Yuan K, Zhang G, Li K, et al. Prostatic
arterial embolization for the treatment of lower urinary tract
symptoms caused by benign prostatic hyperplasia: a comparative
study of medium- and large-volume prostates. BJU Int.
2016;117:155-64. Crossref
15. Gao YA, Huang Y, Zhang R, Yang YD, Zhang Q, Hou M, et al.
Benign prostatic hyperplasia: prostatic arterial embolization versus
transurethral resection of the prostate — a prospective, randomized,
and controlled clinical trial. Radiology. 2014;270:920-8. Crossref