Gemcitabine Plus Nanoparticle Albumin-bound Paclitaxel for Patients with Inoperable Pancreatic Cancer: Experience at a Single Oncology Centre
ORIGINAL ARTICLE
Gemcitabine Plus Nanoparticle Albumin-bound Paclitaxel for Patients with Inoperable Pancreatic Cancer: Experience at a Single Oncology Centre
TY Lee, MHC Lam, KM Cheung, HC Cheng, RKC Ngan, KH Wong
Department of Clinical Oncology, Queen Elizabeth Hospital, Jordan, Hong Kong
Correspondence: Dr TY Lee, Department of Clinical Oncology, Queen Elizabeth Hospital, Jordan, Hong Kong. Email: lukelee2@gmail.com
Submitted: 3 Feb 2019; Accepted: 23 Apr 2019.
Contributors: TYL, HCC, RKCN and KHW designed the study. TYL, MHCL and KMC acquired the data. TYL drafted the manuscript. All authors contributed to data analysis, and had critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As editors of the journal, TY Lee, MHC Lam, and RKC Ngan were not involved in the peer review process. Other authors have no conflicts of interest to disclose.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by the Kowloon Central / Kowloon East institution’s Research Ethics Committee (Ref KC/KE-18- 0177/ER-1) and was conducted in compliance with the Declaration of Helsinki.
Abstract
Objective
To review the outcomes of gemcitabine plus nanoparticle albumin-bound (nab)-paclitaxel for patients with inoperable pancreatic cancer.
Methods
The data of patients treated with this regimen at a single oncology centre in Hong Kong between December 2014 and December 2017 were retrospectively reviewed. Patient data assessed included serial tumour markers (carbohydrate antigen 19-9 and/or carcinoembryonic antigen) and ultrasound, computed tomography, or positron emission tomography-computed tomography scans. The primary objective was to evaluate progression-free and overall survival. The secondary objective was to evaluate the rate of treatment-related toxicities. All adverse events were graded with the Common Terminology Criteria for Adverse Events version 5.
Results
The data of a total of 35 patients were analysed. The median age was 61 years and the majority (77%) had stage IV disease. Histological diagnosis was available in 74% of patients. The median number of cycles received was three. A total of 31% of patients required dose reduction of nab-paclitaxel. Median progression-free survival was 4.9 months (95% confidence interval [CI] = 3.4-6.4), and median overall survival was 7.5 months (95% CI = 5.6-9.4). Overall, 51% of patients received second-line or third-line chemotherapy following disease progression. Grade ≥3 neutropoenia occurred in 29% of patients and febrile neutropoenia in 6%. Grade ≥3 peripheral neuropathy occurred in 9% of patients.
Conclusion
Gemcitabine plus nab-paclitaxel doublet chemotherapy is an effective and safe treatment for inoperable pancreatic cancer. Data from our centre are comparable to literature published to date. However, prognosis remains poor for this disease.
Key Words: Neoplasm metastasis; Pancreatic neoplasms; Paclitaxel; Deoxycytidine/ analogs & derivatives
中文摘要
吉西他濱—納米白蛋白結合型紫杉醇治療不宜手術的胰腺癌患者:單一腫瘤學中心的經驗
李天恩、林河清、張嘉文、鄭海清、顏繼昌、黃錦洪
目的
探討吉西他濱—納米白蛋白結合型紫杉醇對不宜手術的胰腺癌患者的治療效果。
方法
回顧分析2014年12月至2017年12月在香港一所腫瘤中心接受該方案治療的患者數據。評估的患者數據包括系列腫瘤標誌物(碳水化合物抗原19-9和/或癌胚抗原)以及超聲波掃描、電腦斷層掃描或正電子掃描。研究主要評估無惡化存活期和總存活期,其次評估與治療相關的毒性反應的比率。所有不良事件均按照CTCAE第5版進行分級。
結果
共分析35例患者的資料。年齡中位數為61歲,大多數患者(77%)屬第四期。74%患者可進行組織學診斷。化療周期的中位數為3。31%患者須減少納米白蛋白結合型紫杉醇劑量。中位無惡化存活期為4.9個月(95%置信區間3.4-6.4個月),總體存活期中位數為7.5個月(95%置信區間 5.6-9.4個月)。總體而言,51%患者病情惡化後接受二線或三線化療。29%患者出現≥3級中性粒細胞減少症,6%患者出現發熱中性粒細胞減少症。9%患者出現≥3級圍神經病變。
結論
吉西他濱—納米白蛋白結合型紫杉醇治療不宜手術的胰腺癌患者安全和有效。本研究數據與文獻相若,惟疾病的預後仍然欠佳。
INTRODUCTION
Pancreatic cancer is one of the most lethal malignancies,
constituting the sixth leading cause of all cancer deaths
in Hong Kong despite not falling into the top ten
malignancies by incidence.[1] Surgical resection is the
only potentially curative treatment. However, because
presentation of pancreatic cancer is commonly late,
fewer than one-fifth of patients are considered suitable
candidates for pancreatectomy.[2] Even after successful
surgical resection, prognosis remains poor with the
5-year survival after margin-negative surgery being
approximately 10% for node-positive disease and 30%
for node-negative disease.[3]
Systemic chemotherapy is the treatment of choice for
unresectable locally advanced or metastatic pancreatic
cancer. Gemcitabine has been the standard first-line
therapeutic agent since 1997, when it demonstrated
superiority over 5-fluorouracil.[4] Combination regimens
have since been studied and were shown to improve
treatment outcomes, including FOLFIRINOX in the
phase III ACCORD 11 trial,[5] modified FOLFIRINOX,[6]
and gemcitabine plus capecitabine.[7]
In the multinational phase III MPACT trial published
in 2013, the combination regimen gemcitabine plus
nanoparticle albumin-bound (nab)-paclitaxel (GnP)
was demonstrated to be superior to gemcitabine alone,
in terms of overall survival (OS, primary endpoint,
median 8.5 vs. 6.7 months, p < 0.001), progression-free
survival (PFS, median 5.5 vs. 3.7 months, p < 0.001),
and independently reviewed overall response rate (23%
vs. 7%, p < 0.001).[8] Longer-term follow-up data further
confirmed the efficacy of the treatment regimen.[9] Since
then, GnP has become one of several first-line treatment
regimens for patients with good performance status.
However, despite its proven efficacy, combination
treatment is associated with significantly greater toxicities
and costs. The most common grade ≥3 adverse events
with this regimen include leucopoenia, neutropoenia,
peripheral neuropathy, and fatigue.
In the MPACT trial,[8] Asian patients accounted for <2%
of the study population. Data pertaining to the efficacy
and safety of GnP are therefore much needed in order to
support its use in local populations. Therefore, the aim of
this retrospective study was to investigate the outcomes
and safety of GnP for Asian patients with inoperable
pancreatic cancer.
METHODS
All patients with inoperable pancreatic cancer treated
with GnP as first-line or second-line therapy at
Queen Elizabeth Hospital between December 2014
and December 2017 were retrospectively reviewed.
Prescription and dispensing records from the hospital
pharmacy computer system were used to retrieve data on
patients treated with the combination regimen. A total
of 35 patients were identified and included in this study.
Demographic and survival data were extracted from the
hospital’s electronic clinical management system and
medical records.
GnP was given as per departmental protocol. The regimen
consisted of an intravenous infusion of gemcitabine
1000 mg/m2 and nab-paclitaxel 125 mg/m2 on days
1, 8, and 15 every 4 weeks. Primary prophylaxis with
granulocyte colony-stimulating factor (G-CSF) was not
routinely provided. Four patients (11.4%) started with a
lower initial dose (80%-85% dose) after consideration of
the individual functional and disease status. Treatment
was continued until the development of either disease
progression or unacceptable toxicity. Patients were
assessed every 2 weeks during the chemotherapy period
with standardised blood tests. Follow-up visits were
scheduled to take place once every 3 weeks for patients
who received second-line or third-line chemotherapy
after GnP, or every 3 to 8 weeks for patients who did
not receive second-line chemotherapy and those who
completed second-line chemotherapy until death.
Assessment was by means of serial measurements of
tumour markers (carbohydrate antigen 19-9 [CA19-9]
and/or carcinoembryonic antigen) and serial imaging
studies, including ultrasound, computed tomography, or
positron emission tomography–computed tomography
at the physician’s discretion. A biochemical response
was defined as a ≥50% reduction in CA19-9 levels from
baseline. In instances where the serum CA19-9 level was
normal at baseline, carcinoembryonic antigen level and
its subsequent changes were taken into consideration.
Disease response on imaging was assessed using
RECIST version 1.1.[10]
The primary objective was to evaluate the PFS and OS
using the Kaplan-Meier method. PFS was defined as the
time from the date of starting GnP to disease progression
or death from any cause. OS was defined as the time
from the date of starting GnP to the date of death from
any cause. For the purpose of data analysis, the survival
status of all patients was updated on the data cut-off date
31 July 2018. Data from surviving patients would be
censored on the date of last follow-up. The log rank test
was used to test for associations between survival and
demographic or clinical characteristics of the patients.
Hazard ratios were estimated based on the univariate Cox
proportional hazards regression model. Two-tailed tests
were performed and a p value of <0.05 was considered
statistically significant.
The secondary objective was to evaluate treatmentrelated
toxicities. All adverse events were graded
according to CTCAE (Common Terminology Criteria
for Adverse Events) of National Cancer Institute, version
5.0.[11]
Version 4 of the STROBE guidelines for cohort studies
was used in the preparation of this manuscript.[12] The
study was approved by the Kowloon Central/Kowloon
East Research Ethics Committee (Ref KC/KE-18-0177/ER-1) and was conducted in compliance with the
Declaration of Helsinki.
RESULTS
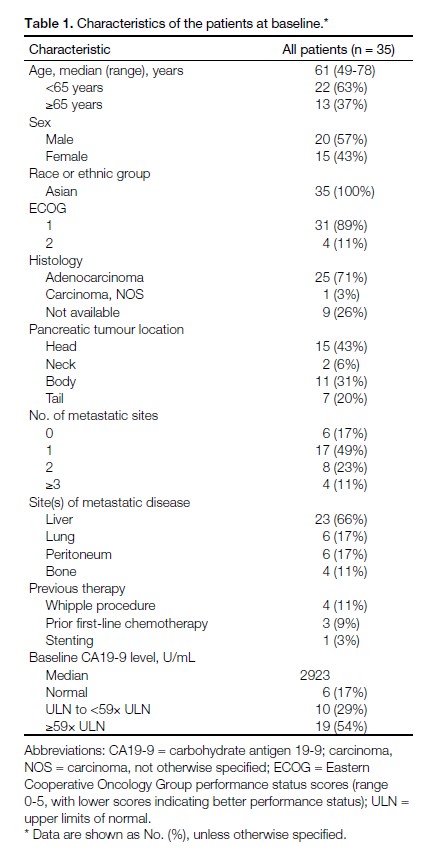
The demographic and clinical characteristics of the
patients are summarised in Table 1. In all, 37% of
patients were aged ≥65 years. Most patients (89%) had a
score of 1 on the Eastern Cooperative Oncology Group
(ECOG) performance scale. Histological diagnosis was
confirmed in 74% of patients. The majority of patients
(83%) had metastatic disease. Approximately 10% of
patients had disease recurrence following prior radical
Whipple procedure.
Table 1. Characteristics of the patients at baseline.
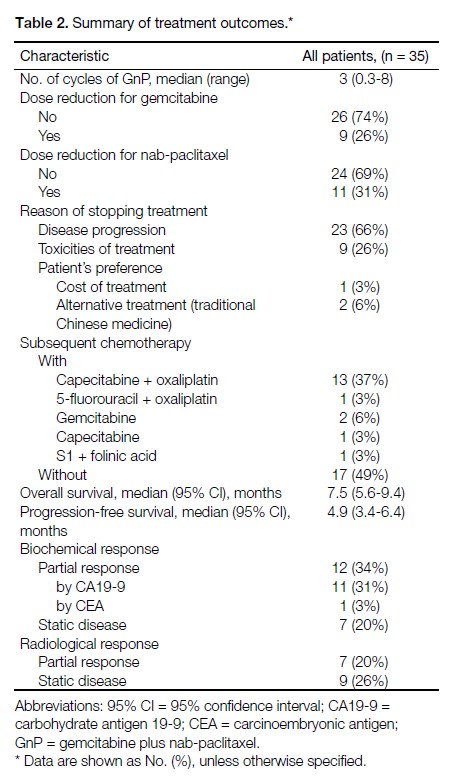
Among the 35 patients included in this study, 32
(91%) received GnP as a first-line regimen, while the
remaining three had had first-line chemotherapy with
either FOLFIRINOX or XELOX. The median number
of GnP chemotherapy cycles received was three and
the maximum number of cycles received was eight.
Around one-third of patients required dose reduction
for nab-paclitaxel due to toxicities. The range of dose
reduction varied between 20% and 40%. In cases where
treatment was aborted, the main reasons included disease
progression (66%) and treatment-related toxicities (26%).
One patient withdrew consent for further treatment due
to financial difficulty. Another two patients opted out of
chemotherapy to receive traditional Chinese medicine
instead based on personal preference. Approximately half
(51%) of all patients received second-line or third-line
chemotherapy subsequent to disease progression, with
the majority (13/18) receiving doublet chemotherapy of
capecitabine plus oxaliplatin. The median duration of
follow-up was 7.5 months (range, 1.4-31.5 months).
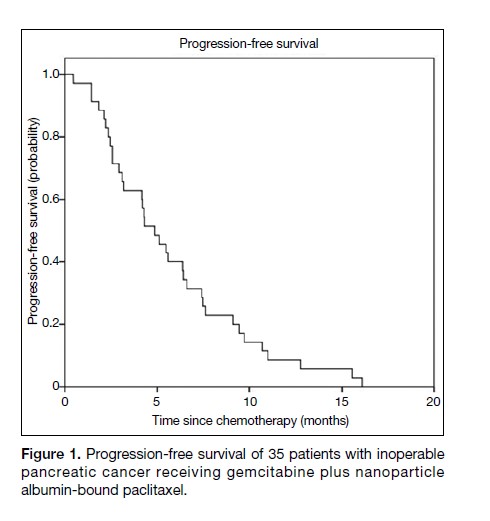
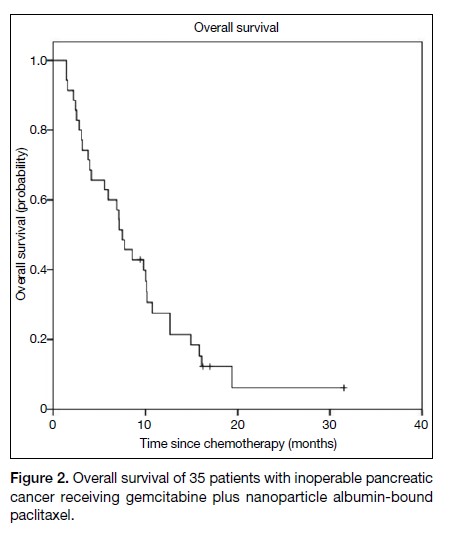
The median PFS was 4.9 months (95% confidence
interval [CI] = 3.4-6.4 months) [Figure 1], and the median
OS was 7.5 months (95% CI = 5.6-9.4) [Figure 2].
Biochemical partial response was noted in 34% of
patients and radiological partial response in 20%. In all,
14% of patients had both biochemical and radiological
partial response. The treatment outcomes are summarised
in Table 2.
Figure 1. Progression-free survival of 35 patients with inoperable pancreatic cancer receiving gemcitabine plus nanoparticle albumin-bound paclitaxel.
Figure 2. Overall survival of 35 patients with inoperable pancreatic cancer receiving gemcitabine plus nanoparticle albumin-bound paclitaxel.
Table 2. Summary of treatment outcomes.
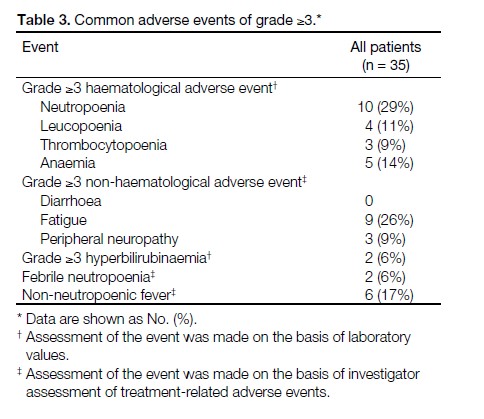
Grade ≥3 neutropoenia and leucopoenia developed
in 29% and 11% of patients, respectively. Febrile
neutropoenia was noted in 6% of patients, whereas nonneutropoenic
fever occurred in 17% of patients. The
most frequently reported grade ≥3 non-haematological
toxicities were fatigue (26%) and peripheral neuropathy
(9%). There were no cases of grade 4 neuropathy. All
significant adverse events are summarised in Table 3.
Table 3. Common adverse events of grade ≥3.
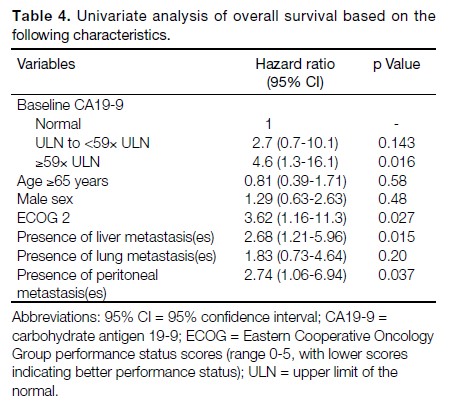
Univariate analysis was used to investigate the
relationship between baseline CA19-9 level and survival.
The OS was found to be lower for those with higher
baseline level of CA19-9 (≥59× upper limits of normal
[ULN]) versus those with normal baseline CA19-9
(hazard ratio [HR] = 4.6, 95% CI = 1.3-16.1, p = 0.016).
The results are summarised in Table 4.
Table 4. Univariate analysis of overall survival based on the
following characteristics.
Further univariate analysis of OS based on different
demographic features was performed using the log rank
test. It was found that patients with ECOG 2 had poorer
survival (HR = 3.62, 95% CI = 1.16-11.3, p = 0.027).
The presence of hepatic metastatic disease (HR = 2.68,
95% CI = 1.21-5.96, p = 0.015) or peritoneal metastases
(HR = 2.74, 95% CI = 1.06-6.94, p = 0.037) were
associated with worse OS. The results are summarised
in Table 4.
DISCUSSION
Systemic combination chemotherapy is the current
standard treatment for patients with unresectable locally
advanced or metastatic pancreatic cancer who have
good performance status. GnP has been incorporated
into various international guidelines, including ESMO,[13]
NCCN,[14] and NICE[15] guidelines.
The current guidelines at our institution offer a number
of chemotherapy regimens to be used as first-line
therapy for advanced or metastatic pancreatic exocrine
carcinoma. These include single-agent gemcitabine
or S-1, combination chemotherapy regimens such as
gemcitabine plus capecitabine, GnP, and FOLFIRINOX.
FOLFIRINOX has been shown to confer a significant
improvement in survival at the expense of increased
toxicity.[16] Therefore, FOLFIRINOX is generally offered
to a highly selected patient group, consisting primarily
of young fit patients with very good performance status.
Most of our patients with ECOG 1-2 are offered less
intensive combination chemotherapy regimens of either
gemcitabine plus capecitabine, or GnP. nab-Paclitaxel is
a self-financed item in public hospitals in Hong Kong;
therefore, only those patients who can afford nabpaclitaxel
will receive GnP, and others will be given
gemcitabine plus capecitabine. Single-agent gemcitabine
or S-1 is often given to systemically more frail patients.
In this retrospective study, only patients receiving GnP
therapy were reviewed.
The data on PFS and OS from this retrospective study are
comparable to the published outcomes of the MPACT
trial. The median PFS was 4.9 months (95% CI = 3.4-6.4)
in this study, compared to 5.5 months (95% CI = 4.5-5.9)
in the MPACT trial.8 The median OS was 7.5 months
(95% CI = 5.6-9.4) in this study, versus 8.5 months
(95% CI = 7.9-9.5) in the MPACT trial. This finding is
also consistent with another phase II study performed
in a Chinese population, in which the median PFS was
5.5 months (95% CI = 5.3-7.2) and median OS was
9.2 months (95% CI = 7.6-11.1).[17]
Advanced age itself is not an absolute contra-indication
for combination chemotherapy, and such regimens are
generally well tolerated in older patients. The oldest
patient in this study was aged 78 years, with an ECOG
performance status of 1, who received a total of eight
cycles of GnP without any need for dose reduction.
This finding also concurs with the MPACT trial,8 which
included patients aged >80 years.
The doublet regimen GnP was well tolerated by
patients in the current study. Although primary G-CSF
prophylaxis was not routinely given to our patients, the
febrile neutropoenia rate was 6%. This is similar to the
result of the MPACT trial (3%),8 in which 26% of patients
received G-CSF. No new safety issues were observed.
The baseline CA19-9 level was elevated in approximately
83% of patients, and the majority (54%) had a level ≥59×
ULN. CA19-9 has historically been widely used as a
surrogate marker for disease progression during followup
assessments after radical surgery or during ongoing
chemotherapy.[18] However, there has yet to be a universal
consensus as to the extent to which CA19-9 may be used
as a surrogate marker for response evaluation during
chemotherapy.[19] Serial monitoring of CA19-9 level was
performed in our patients, and we defined a biochemical
response as a 50% reduction from baseline, as proposed in
the literature.[20,21,22] Based on this definition, a biochemical
response was observed in 34% of our patients.
The OS was found to be lower for those with higher
baseline levels of CA19-9 (≥59× ULN) than those with
normal baseline levels. This observation was consistent
with previous studies, which suggested higher baseline
CA19-9 levels to be generally associated with worse
clinical outcomes.[23,24,25]
Univariate analysis showed that patients with worse
pretreatment performance status (ECOG 2), presence
of liver metastasis(es), or peritoneal disease had poorer
OS. These findings were consistent with prior reported
data.[26,27,28] However, owing to the limited sample size in
this study, further multivariate analysis was not possible.
There are a number of limitations in the current study.
Firstly, the sample size in this retrospective study was
relatively small, as many of our patients in the public
health care system cannot afford the self-financed drug
(nab-paclitaxel) or had co-morbidities that rendered
them unfit for doublet chemotherapy. This may have
led to selection bias as only relatively well-off patients,
who might inherently be more health-cautious, have
better performance status, greater social support, and
earlier access to healthcare could afford the treatment.
Because of the relatively small sample size, the results
of univariate analysis should be interpreted with caution
owing to the limited statistical power. Similarly, further
descriptive analysis on the OS according to the secondor
third-line regimens was difficult owing to the small
sample size, whereby most regimens of the subsequent
chemotherapy were given to only one or two patients.
Lastly, three patients in the study received GnP as a
second-line regimen. These patients might therefore
have experienced lower response rates and worse
survival outcomes that could potentially have an adverse
effect on the study results.[29,30]
CONCLUSION
GnP is an effective and safe first-line treatment for Asian
patients with inoperable pancreatic cancer.[29,31] Data from
the present study are consistent and comparable with
the literature published to date. Higher baseline CA19-9
level was a negative prognostic factor. Future studies
should focus on determining the optimal combination
regimen and sequence of treatment for patients with
inoperable or metastatic pancreatic cancer.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR Government. Available from: https://www3.ha.org.hk/cancereg/topten.html. Accessed 1 Aug 2018.
2. Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-65. Crossref
3. Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185-91. Crossref
4. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-13. Crossref
5. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-25. Crossref
6. Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017-23. Crossref
7. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-8. Crossref
8. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703. Crossref
9. Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2). pjj:dju413. Crossref
10. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. Crossref
11. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 2 Mar 2020.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. Crossref
13. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol. 2015;26 Suppl 5:v56-68. Crossref
14. National Comprehensive Cancer Network. NCCN Guidelines. Pancreatic Adenocarcinoma, version 3. 2017, NCCN Clinical Practice Guidelines in Oncology. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 2 Mar 2020.
15. National Institute for Health and Care Excellence. NICE Guidance. Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer. Technology appraisal guidance [TA476]. Available from: https://www.nice.org.uk/guidance/ta476. Accessed 2 Mar 2020.
16. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:181-25. Crossref
17. Xu R, Yu X, Hao J, Wang L, Pan H, Han G, et al. Efficacy and safety of weekly nab-paclitaxel plus gemcitabine in Chinese patients with metastatic adenocarcinoma of the pancreas: a phase II study. BMC Cancer. 2017;17:885. Crossref
18. Safi F, Schlosser W, Falkenreck S, Beger HG. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253-9.
19. Stemmler J, Stieber P, Szymala AM, Schalhorn A, Schermuly MM, Wilkowski R, et al. Are serial CA 19-9 kinetics helpful in predicting survival in patients with advanced or metastatic pancreatic cancer treated with gemcitabine and cisplatin? Onkologie. 2003;26:462-7. Crossref
20. Reni M, Cereda S, Balzano G, Passoni P, Rognone A, Fugazza C, et al. Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer. 2009;115:2630-9. Crossref
21. Ishii H, Okada S, Sato T, Wakasugi H, Saisho H, Furuse J, et al. CA 19-9 in evaluating the response to chemotherapy in advanced pancreatic cancer. Hepatogastroenterology. 1997;44:279-83.
22. Saad ED, Machado MC, Wajsbrot D, Abramoff R, Hoff PM, Tabacof J, et al. Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer. 2002;32:35-41. Crossref
23. Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255-64. Crossref
24. Bauer TM, El-Rayes BF, Li X, Hammad N, Philip RA, Shields AF, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119:285-92. Crossref
25. Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Rubstaller T, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomized controlled trial. Lancet Oncol. 2008;9:132-8. Crossref
26. Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-16. Crossref
27. Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase 3 trial of gemcitabine plus tipifarib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22;1430-8. Crossref
28. Le N, Sund M, Vinci A. GEMS collaborating group of Pancreas 2000. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig Liver Dis. 2016;48:223-30. Crossref
29. Citterio C, Baccini M, Orlandi E, Di Nunzio C, Cavanna L. Secondline chemotherapy for the treatment of metastatic pancreatic cancer after first-line gemcitabine-based chemotherapy: a network metaanalysis. Oncotarget. 2018;9:29801-9. Crossref
30. Dadi N, Stanley M, Shahda S, O’Neil BH, Sehdev A. Impact of nab-paclitaxel-based second-line chemotherapy in metastatic pancreatic cancer. Anticancer Res. 2017;37:5533-9. Crossref
31. Tahara J, Shimizu K, Otsuka N, Akao J, Takayama Y, Tokushige K. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2018;82:245-50. Crossref